INTRODUCTION
It has now been over 20 years since the Institute of Medicine published the paradigm-changing report “To Err is Human,” which concluded that as many as 98,000 deaths occurred in hospitals each year due to errors in care.1 Although the exact figure of anesthesia-related mortality is controversial, there is no question that our specialty has made remarkable gains in improving patient safety over the past two decades due to improvements in training, equipment, and standardized protocols. Safe management of orthotopic liver transplantation (OLT) patients, however, continues to be one of the most challenging perioperative cases for anesthesia professionals. OLT involves multidisciplinary collaboration, which includes surgical, anesthesia, nursing teams, as well as perfusionist and other specialized teams (e.g., blood bank, dialysis, and ICU). The procedure is technically complex, and the intraoperative course is associated with hemodynamic instability, acid-base and metabolic derangements, coagulation complications, wide fluid shifts, and is still associated with more intraoperative deaths than any other surgical procedure.2
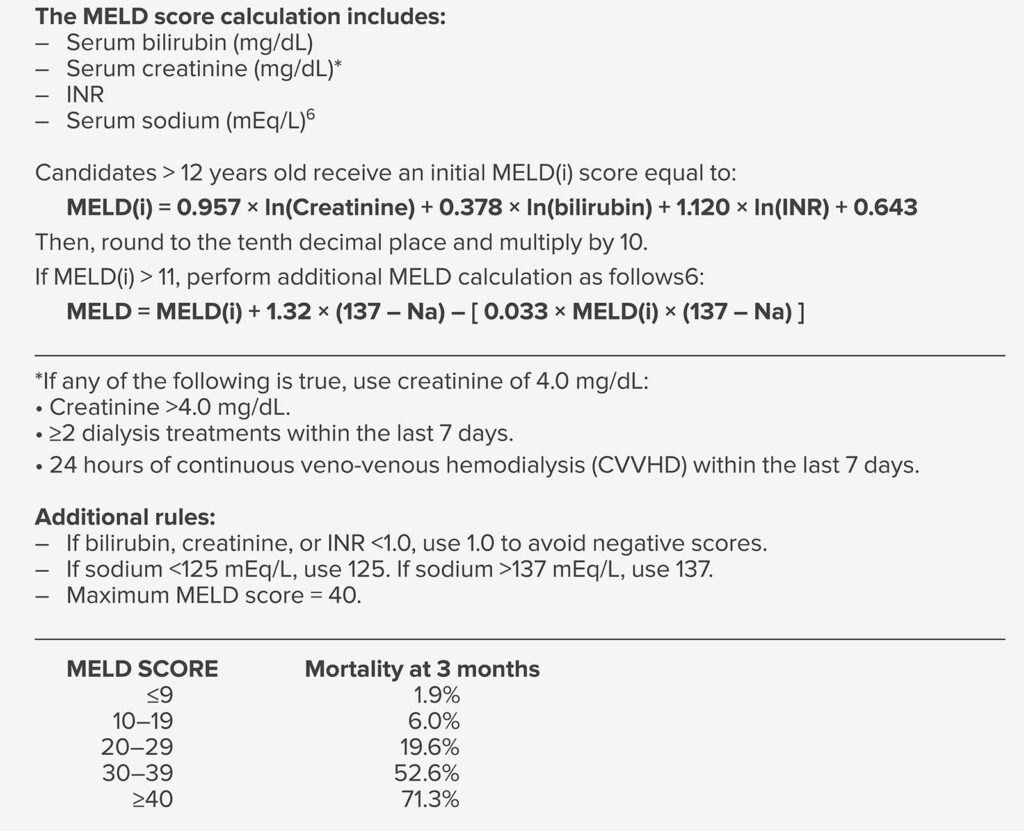
Although success in liver transplantation has led to more liver transplantations being performed each year, the number of donated organs has reached a plateau. Adoption of the Model for End-Stage Liver Disease (MELD) based system in 2002 has led transplantation that prioritizes the “sickest first” (Table 1). Patients with high MELD scores are expected to have abnormal levels of bilirubin, creatinine, INR, sodium, or a combination of each (see Table 1). Abnormalities in each of these MELD components is associated with high perioperative risk in previous studies.3 This has a profound effect on patients presenting for liver transplantation, particularly in populated areas where there are more transplantation centers. Such evolution in patient selection and the increasing severity of disease in patients at liver transplantation has posed many perioperative challenges to physicians who care for these patients.4 Patients who present for liver transplant today have higher MELD scores and more advanced liver disease, more advanced age and preoperative comorbidities, more renal and electrolyte abnormalities, and higher requirements for intraoperative transfusions and vasopressors than patients who presented for liver transplant twenty years ago in the pre-MELD era.3
Table 1: MELD Score Components and 3 Month Mortality Prediction5
Decisions on which patients are included on transplant waiting lists today may also be further distorted by intense competition in populated areas where there are more transplant centers. Our institution, the University of Southern California (USC), is a high-volume transplant center in the Los Angeles metropolitan area where there are three transplant centers located within a 20-mile radius. The combined volume of liver transplants performed at both Keck Hospital and Children’s Hospital Los Angeles (CHLA) last calendar year adds up to the second-busiest liver transplant program in the nation according to data from the United Network for Organ Sharing. With local competition and prestige at stake, it is understood that centers may be motivated to perform more transplants, resulting in more challenging patients on the waiting list. Nevertheless, liver transplantation surgery today challenges the full capacities of the systems and processes involved in patient safety. This article describes a few of the processes developed at our institution and how they contribute to patient safety in a field that is becoming increasingly more challenging.
DEVELOPMENT OF DESIGNATED LIVER TRANSPLANT ANESTHESIA TEAMS
In 2011 the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) required all transplant centers appoint a director of liver transplant anesthesia.7 This declaration by a nationally recognized governing and regulatory body was the first step in acknowledging liver transplant anesthesiology as an independent subspeciality of anesthesiology. From here, the development of liver transplant anesthesia teams and transplant anesthesia fellowships followed. The value of dedicated anesthesia teams was further supported by evidence showing dedicated transplant teams reduced transfusion, time of postoperative ventilation, length of intensive care unit stay, and perioperative mortality.8
In addition to providing clinical care, members of the liver transplant anesthesia team are involved with various perioperative transplant surgery functions such as patient selection committees. The multidisciplinary committee includes transplant coordinators, surgeons, hepatologist, nephrologist, infectious disease specialists, anesthesia professionals, and social workers. At these weekly committees, we discuss the patient’s liver history and other medical problems, and then discuss issues of social support, substance abuse, and finances. From here, the decision-making process involves an ordered review of possible reasons for exclusion. Routine involvement of the anesthesia team in the selection process allows us to formally evaluate patients before they present for liver transplant. If needed, patients may be referred to our preoperative clinic to allow a member of the anesthesia team to further evaluate the patient’s physical status for liver transplant.
PRE-TRANSPLANT AND ABO VERIFICATION
Due to the complexity of coordination in transplant, additional safety verification processes are involved. Verification of blood type at multiple and defined points in the transplantation process ensures the safety and compatibility of our transplant donors and recipients. Vital information such as organ type, donor and recipient ID, donor and recipient ABO blood type, and recipient date of birth and medical record number will be verified during living donor registration, prior to living donor organ recovery, prior to organ receipt in the operating room (if recipient surgery begins prior to organ receipt in the operating room), and upon organ receipt in the operating room. Verification is required by two licensed health care professionals. If the recipient will begin prior to organ receipt in the operating room, verification must occur either prior to induction of anesthesia or prior to incision. Additionally, blood components are scanned using an electronic verification system intraoperatively. OLT can require up to 10 times as many units of blood products as a heart transplant.9 Verification via barcode scanning allows for one-person verification and increases workflow efficiency while minimizing transfusion errors related to misidentification.
EVOLUTION OF PATIENT BLOOD MANAGEMENT PROGRAM AT KECK USC
Although blood transfusions are a lifesaving therapy for some patients, transfusions were identified as 1 of the top 5 overused procedures by the Joint Commission’s Overuse Summit in 2012.10 The Transfusion Free Surgery and Patient Blood Management Program at Keck USC was initially developed in 1997 to serve the specific needs of the Jehovah’s Witness (JW) community. Our center gained national recognition after our transplantation team performed the first successful transfusion-free living donor liver transplantations in 1999 using techniques like acute normovolemic dilution. From 1999–2004, 27 liver transplantations, consisting of both living donors and deceased donors, were performed in JW patients at the USC-University Hospital.11 The relative success of liver transplantation in JW patients has allowed the opportunity to critically assess the use of blood products in surgery at large. What started at Keck USC as a narrowly focused initiative has expanded into a much broader mainstream program that serves non-JW patients. This development was driven by the concept that minimizing blood product administration enhances patient safety and reduces the cost and length of hospital stay. Higher rates of transfusion have been associated with increased length of hospital stay, higher rates of infection, graft failure, and mortality.12
Given that most of the evidence supporting a restrictive transfusion strategy has been published in the past decade, patient blood management programs have only recently gained popularity. Efforts to reduce overuse of transfusions through patient blood management programs at our institution have been successful. Currently, a retrospective study is being conducted during the writing of this article. Preliminary data collection reports a ~20% decrease in RBCs, platelets, and plasma utilization for liver transplant cases in 2021 compared to 2020, despite an increase in cases. Our reduction in transfusions in LT was a result of several key interventions implemented which will be discussed. First, a hospital-wide campaign to educate and promote change to the culture of liberal blood utilization practice was implemented. One successful strategy was adopting the “Why give 2 when 1 will do?” Choosing Wisely campaign to reduce orders of multi-unit RBC transfusions.13 A widespread communication effort followed in our hospital newsletters and on computer screensavers to encourage single unit transfusions.
Another intervention that changed our transfusion practice at our institution was implementation of intraoperative thromboelastography (TEG). Despite the lack of large randomized clinical studies, viscoelastic tests have been a critical armamentarium for hemostatic control in liver transplantation since Thomas Starzl, MD, performed the first LT the 1960s.14 Many transplant institutions have adopted viscoelastic tests like TEG in their clinical practice. However, it was only recently that TEG at our center was made expedient and efficient, both intraoperatively and postoperatively in the ICU, to allow for a rapid and real-time, qualitative assessment of the different components of hemostasis. Lastly, and perhaps most importantly, we believe our success in blood management is a result of improved communication between liver anesthesia and surgical teams over the progress of the case. For example, improved communication and use of TEG has allowed us to better distinguish surgical bleeding from bleeding due to coagulopathy, which helped reduce intraoperative transfusions. Overall, we hope to demonstrate how multidisciplinary teams can significantly reduce total blood product utilization in OLT.
THE ROLE OF INTRAOPERATIVE HEMODIALYSIS (HD) IN LIVER TRANSPLANTATION
Liver transplantation for patients with renal dysfunction is frequently complicated by major fluid shifts, acidosis, and electrolyte and coagulation abnormalities that require large volumes of blood products and crystalloid solutions. In the early years of OLT, liver transplant anesthesia professionals used to manage cases with renal failure with strict fluid management and continuous metabolic adjustments without the help of intraoperative hemodialysis (HD). However, despite vigilant monitoring of the patient’s hemodynamics and metabolic derangements, the intraoperative course in many cases was complicated by the overwhelming fluid and metabolic changes that occur in patients with renal impairment or failure. The rationale behind the use of intraoperative renal replacement therapy during liver transplant for patients with renal failure is that the surgery is usually complicated by major hemodynamic instability, coagulation abnormalities, and metabolic derangements. At our center, liver transplant continues to be the only case that routinely uses intraoperative HD in anesthetic management.15 Our institution was one of the first to demonstrate the safety and feasibility of intraoperative HD and adopt its use in the critically ill with high MELD (mean ~37) scores undergoing LT.16
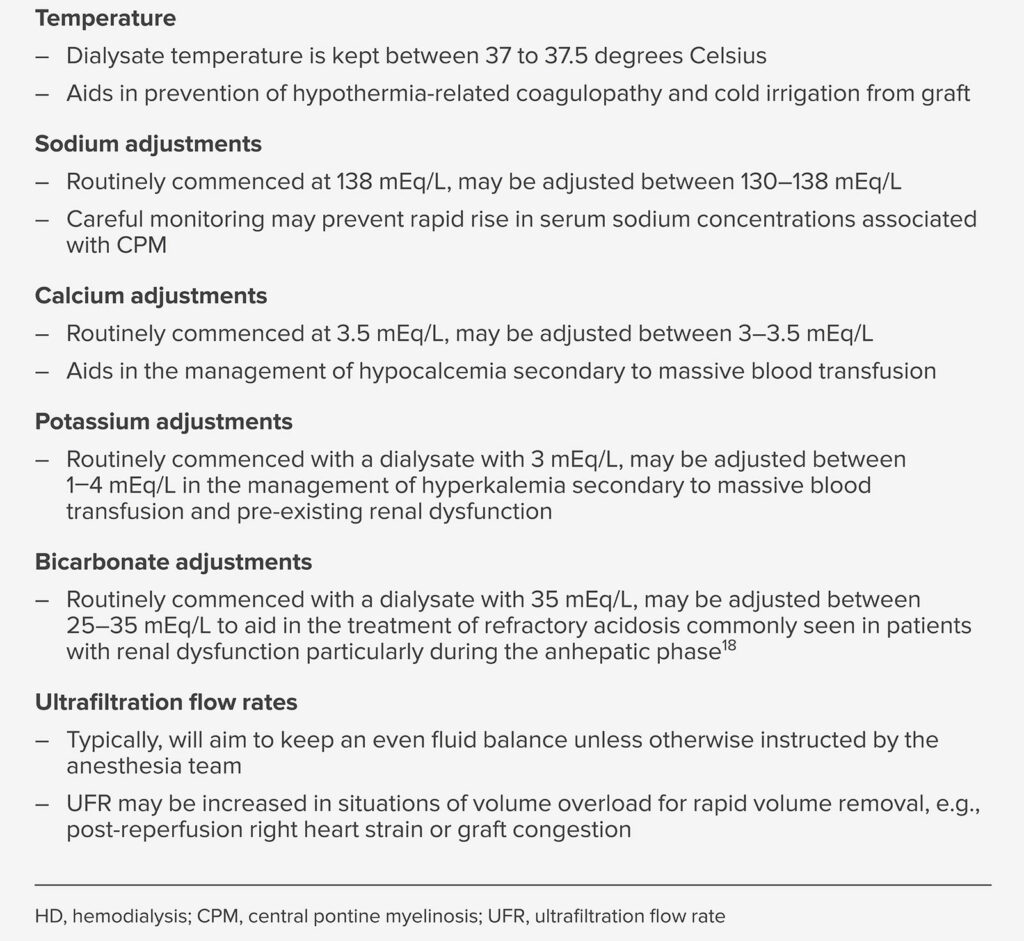
The decision whether to use intraoperative HD during OLT is a collaborative one between the surgeon, anesthesia team, and nephrologist depending on the degree of renal dysfunction and the overall clinical picture including the need for postoperative renal replacement therapy. Generally, intraoperative HD will be used on patients with Glomerular Filtration Rate < 60 ml/min or serum creatinine >1.4 mg/dL. For those without permanent dialysis access, a dual-lumen HD catheter is inserted into the internal jugular, subclavian, or femoral vein. Prior to surgery, the nephrologist decides on the concentration of sodium, calcium, potassium, and bicarbonate in the dialysate solution for each patient based on their laboratory values. During the operation, the HD nurse works in close consultation with the anesthesia team. Half-hourly to hourly blood gases are drawn to help guide changes in the dialysate as needed (mainly adjustments to the bicarbonate and potassium levels).17 The use of intraoperative HD aids in the management of temperature, acidosis, hyperkalemia, and volume overload, all of which are associated with intraoperative morbidity and mortality in patients undergoing liver transplant.15 The anesthesia professional is acquainted with the various treatment options available (Table 2). With a thorough evaluation, monitoring, and continuous appropriate interventions, intraoperative HD can be used safely and effectively in critically ill patients undergoing LT with high MELD scores and renal dysfunction.
Table 2: Summary of Treatment Variations During Intraoperative HD
CONCLUSION
The changing face of patients presenting for liver transplantation today has posed many challenges to the systems and processes involved in patient safety. In this article, we reviewed a few of the processes implemented at our center that have allowed us to improve safety measures and outcomes in critically ill, high MELD patients undergoing liver transplant. In order to continue to improve patient safety in liver transplantation, more comprehensive data and studies are required to further characterize the evolving safety challenges in liver transplantation today.
References
- Kohn LT, Corrigan J, Donaldson MS. To err is human : building a safer health system. National Academy Press; 2000, p. 87. PMID: 25077248.
- Butt Z, Parikh ND, Skaro AI, et al. Quality of life, risk assessment, and safety research in liver transplantation: new frontiers in health services and outcomes research. Curr Opin Organ Transplant. Jun 2012;17:241–247. PMID: 22476225.
- Xia VW, Taniguchi M, Steadman RH. The changing face of patients presenting for liver transplantation. Curr Opin Organ Transplant. Jun 2008;13:280–284. PMID: 18685318.
- Zarrinpar A, Busuttil RW. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol. July 2013;10:434–440. PMID: 23752825.
- Kremers WK, van IJperen M, Kim WR, et al. MELD score as a predictor of pretransplant and posttransplant survival in OPTN/UNOS status 1 patients. Hepatology. 2004;39:764–769. PMID: 14999695.
- OPTN 2016 MELD Policy Changes 2016. https://optn.transplant.hrsa.gov/news/meld-serum-sodium-policy-changes/. https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf. Both accessed December 14, 2022.
- Nguyen-Buckley C, Wray CL, Zerillo J, et al. Recommendations from the Society for the Advancement of Transplant Anesthesiology: liver transplant anesthesiology fellowship core competencies and milestones. Semin Cardiothorac Vasc Anesth. 2019;23:399–408. PMID: 31402752.
- Hevesi ZG, Lopukhin SY, Mezrich JD, et al. Designated liver transplant anesthesia team reduces blood transfusion, need for mechanical ventilation, and duration of intensive care. Liver Transpl. 2009;15:460–465. PMID: 19399745.
- Nedelcu E, Wright MF, Karp S, Cook M, et al. Quality improvement in transfusion practice of orthotopic liver transplantation reduces blood utilization, length of hospital stay, and cost. Am J Clin Pathol. 2019;151:395–402. PMID: 30535323.
- Sadana D, Pratzer A, Scher LJ, et al. Promoting high-value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med. 2018;178:116–122. PMID: 29159367.
- Jabbour N, Gagandeep S, Mateo R, et al. Transfusion free surgery: single institution experience of 27 consecutive liver transplants in Jehovah’s Witnesses. J Am Coll Surg. 2005;201:412–417. PMID: 16125075.
- Tokin C, Almeda J, Jain S, et al. Blood-management programs: a clinical and administrative model with program implementation strategies. Perm J. Winter 2009;13:18–28. PMID: 21373242.
- Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “Why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164. PMID: 27624209.
- Sakai T. Viscoelastic testing in liver transplantation. Transfusion. 2020;60 Suppl 6:S61–S69. PMID: 33089935.
- Sedra AH, Strum E. The role of intraoperative hemodialysis in liver transplant patients. Curr Opin Organ Transplant. 2011;16:323–325. PMID: 21543980.
- Nadim MK, Annanthapanyasut W, Matsuoka L, et al. Intraoperative hemodialysis during liver transplantation: a decade of experience. Liver Transpl. 2014;20:756–764. PMID: 24634344.
- Henson A, Carpenter S. Intra-operative hemodialysis during liver transplantation: an expanded role of the nephrology nurse. Nephrol Nurs J. 2010;37:351–353, 356; quiz 354. PMID: 20830942.
- Vitin A, Muczynski K, Bakthavatsalam R, et al. Treatment of severe lactic acidosis during the pre-anhepatic stage of liver transplant surgery with intraoperative hemodialysis. J Clin Anesth. 2010;22:466–472. PMID: 20868970.