Authors: Nair A et al
Cureus 17(6): e86691. doi:10.7759/cureus.86691 June 24, 2025
Abstract
Obese patients undergoing bariatric and metabolic surgeries require tailored perioperative pain management. This review aimed to compare the analgesic efficacy and safety of two adjuncts used in general anesthesia (GA), remifentanil and dexmedetomidine, in this patient population. Using relevant keywords, we searched PubMed, Scopus, the Cochrane Library, and ClinicalTrials.gov, identifying five randomized controlled trials for a qualitative systematic review and quantitative meta-analysis. The RoB 2 tool was used to assess the risk of bias, and the meta-analysis was conducted using RevMan version 5.4. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was employed to evaluate the overall quality of evidence. Trial Sequential Analysis (TSA) was used to confirm significant findings. The overall risk of bias was low, and the GRADE quality ranged from moderate to low. Twenty-four-hour opioid consumption and pain scores in the recovery room were comparable between the two groups (mean difference (MD): 0.23; 95% CI: -1.42 to 1.89, P = 0.78; and MD: 0.04; 95% CI: -0.48 to 0.57, P = 0.87, respectively). Postoperative nausea and vomiting (PONV) was significantly lower in the dexmedetomidine group (OR: 2.55; 95% CI: 1.60 to 4.07, P < 0.0001), a finding confirmed by TSA. However, the cumulative sample size represented only 82.5% of the required information size. Overall heterogeneity was low to moderate. Based on the findings of this review, analgesic efficacy, measured by 24-hour opioid consumption and recovery room pain scores, appears comparable between remifentanil and dexmedetomidine. However, the incidence of PONV was significantly lower in the dexmedetomidine group. Further studies are warranted to identify the most suitable adjunct to GA in this high-risk patient population.
Introduction & Background
Obesity affects pharmacokinetics, pharmacodynamics, and respiratory physiology, presenting specific challenges in anesthesia management. Altered drug distribution and opioid elimination in obese patients can lead to respiratory depression and prolonged sedation if not used judiciously. This necessitates monitoring in high-dependency areas, potentially delaying recovery and early mobilization [1]. The volume of distribution for lipophilic opioids, such as fentanyl, increases due to excess adipose tissue, leading to drug accumulation and prolonged effects [2]. In contrast, hydrophilic opioids like remifentanil exhibit a more predictable pharmacokinetic profile in patients undergoing bariatric and metabolic surgeries, as their rapid metabolism remains unaffected by body fat [3].
Dexmedetomidine, a selective alpha-2 adrenergic agonist, possesses analgesic, sedative, and opioid-sparing properties. Its sympatholytic effects lower blood pressure and heart rate without causing significant respiratory depression, making it particularly beneficial for obese patients [4]. By reducing opioid requirements and maintaining airway reflexes and spontaneous breathing, dexmedetomidine facilitates opioid-sparing anesthesia and minimizes the risk of respiratory depression [5].
Remifentanil, a unique opioid metabolized by nonspecific esterases, offers a rapid and predictable onset and offset of action due to its quick clearance [6]. Although its ultra-short half-life accelerates postoperative recovery, alternative analgesia is required postoperatively [7]. In patients with obstructive sleep apnea (OSA) or opioid sensitivity, dexmedetomidine enhances postoperative pain management, reduces opioid requirements, and improves the quality of recovery. Dexmedetomidine is primarily metabolized by hepatic enzymes, and its clearance may be affected by changes in regional blood flow and hepatic function in obesity. Since OSA is highly prevalent in obese patients, opioid-induced respiratory depression remains a major concern [8]. Despite its rapid metabolism, remifentanil still carries a limited but present risk of respiratory depression. In contrast, dexmedetomidine reduces opioid requirements, preserves airway reflexes, and supports spontaneous breathing, offering an added advantage in minimizing respiratory complications.
Several studies have compared opioids with opioid-sparing strategies, such as dexmedetomidine, in patients undergoing bariatric surgeries [9,10]. This review aimed to compare the analgesic efficacy of remifentanil and dexmedetomidine, along with other outcomes including pain scores, adverse events, and anesthesia and surgical duration, when used as adjuncts to general anesthesia (GA) in patients undergoing bariatric and metabolic surgeries.
Review
Materials and methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review was prospectively registered with PROSPERO (CRD420251002300) at https://www.crd.york.ac.uk/PROSPERO.
Search Strategy
We searched major databases (PubMed, Scopus, Embase, Cochrane Library) and an additional clinical trial registry (ClinicalTrials.gov). We sought randomized controlled trials (RCTs) comparing remifentanil with dexmedetomidine as adjuncts to anesthesia using relevant keywords, covering the period from January 2000 to February 2025. The details of the search strategy and truncations are provided in Table 1. The primary outcome was the comparison of 24-hour opioid consumption. The secondary outcomes included the comparison of adverse events like postoperative nausea/vomiting (PONV), hemodynamic and respiratory adverse events, pain scores, and surgical and anesthesia time.
Eligibility Criteria
To identify studies that are relevant, we employed a structured Population, Intervention, Control, Outcome, and Study (PICOS) design. The quantitative analysis was confined to RCTs. Studies performed in languages other than English, research involving animals, studies for which full texts were not available, systematic reviews, literature reviews, scoping reviews, case reports, series, editorials, and conference abstracts were excluded.
Population: All adult patients undergoing elective bariatric and metabolic surgeries under GA were included.
Intervention: Use of intraoperative infusion of remifentanil.
Control: Use of intraoperative infusion of dexmedetomidine.
Outcome: Analgesic efficacy, adverse events, surgery, and anesthesia time.
Study: RCTs
Data Extraction
Two authors (AN and TM) independently screened the identified studies based on the inclusion and exclusion criteria. A third author (HE) was asked to resolve any disagreements regarding study inclusion. Once the articles were finalized, their details were summarized in a table under the following headings: author and year of publication, country, type of study, number of participants per intervention arm, description of intervention and control, and primary and secondary outcomes.
Methodological Assessment
Using the Risk of Bias (ROB2) tool and the Cochrane Intervention System Evaluation Manual (Cochrane Handbook for Systematic Reviews of Interventions), two authors (AN and TM) independently reviewed the methodological quality of the articles that fulfilled the inclusion criteria [11]. If these two researchers disagreed on the methodological evaluation, a third researcher (HE) was involved to resolve the discrepancy and make a decision. Bias resulting from the randomization process, bias resulting from deviations from the intended intervention, bias resulting from missing outcome data, bias in outcome measurement, and bias in the selection of the reported result were all included in the methodological assessment (ROB2).
Strength of Quality Across All Trials
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) guidelines were adopted to assess the methodological quality of the evidence across pooled outcomes [12]. The study design, ROB, consistency, directness, precision, and additional factors including confounding, large effect size, and publication bias were taken into account when establishing the quality of evidence for pooled outcomes. The certainty of the evidence was categorized as follows: (1) high quality, additional research is improbable to alter the confidence in the estimate of effect; (2) moderate quality, additional research is likely to affect the estimate’s confidence significantly and may alter it; (3) low quality, additional research is highly likely to alter the estimate; or (4) very low quality, the estimate is uncertain.
Quantitative Meta-Analysis
We used Review Manager software (RevMan 5.4) by the Cochrane Collaboration for conducting the meta-analysis [13]. The binary variables were reported using OR and 95% CI, and the continuous variables with mean difference (MD) and 95% CI. The effect estimates were derived from forest plot analysis comparing similar groups. A p-value less than 0.05 was regarded as statistically significant. Inconsistencies were evaluated using the χ² and I² statistics to assess the clinical heterogeneity in the included studies, and p < 0.10 and I² > 50% indicated that χ² had statistical differences [14]. A fixed-effects model was utilized if the study results showed heterogeneity, i.e., I² < 50% and p > 0.10. The random-effects model was utilized for the meta-analysis in all other cases [15]. For comparison purposes between the trials, different opioids were converted to IV morphine equivalent (https://www.mdcalc.com/calc/3947/opiate-conversion-calculator).
Sensitivity Analysis
Each study was sequentially removed from the analysis to rule out any particular study exerting greater influence on the interpretation of the results [16]. A funnel plot was constructed to determine if there was publication bias [17].
Trial Sequential Analysis (TSA)
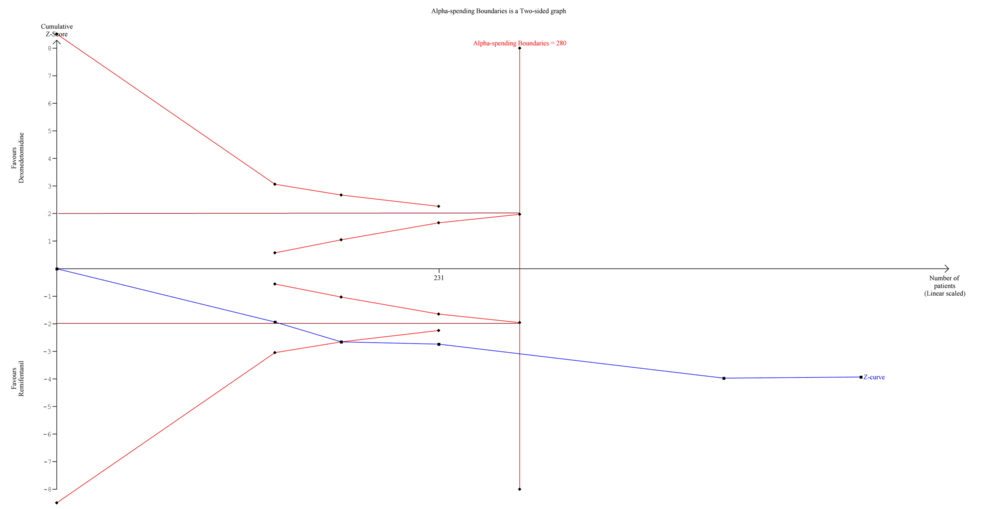
We planned a TSA for those outcomes that were statistically significant in quantitative meta-analysis using the TSA Module version 0.9.5.10 (Copenhagen Trial Unit, Denmark) to calculate the required information size (RIS) and see if our findings were conclusive. The DerSimonian-Laird (DL) method was used to create the cumulative Z-curve. The TSA was carried out to keep the overall risk of a Type I error at 5% [18].
Results
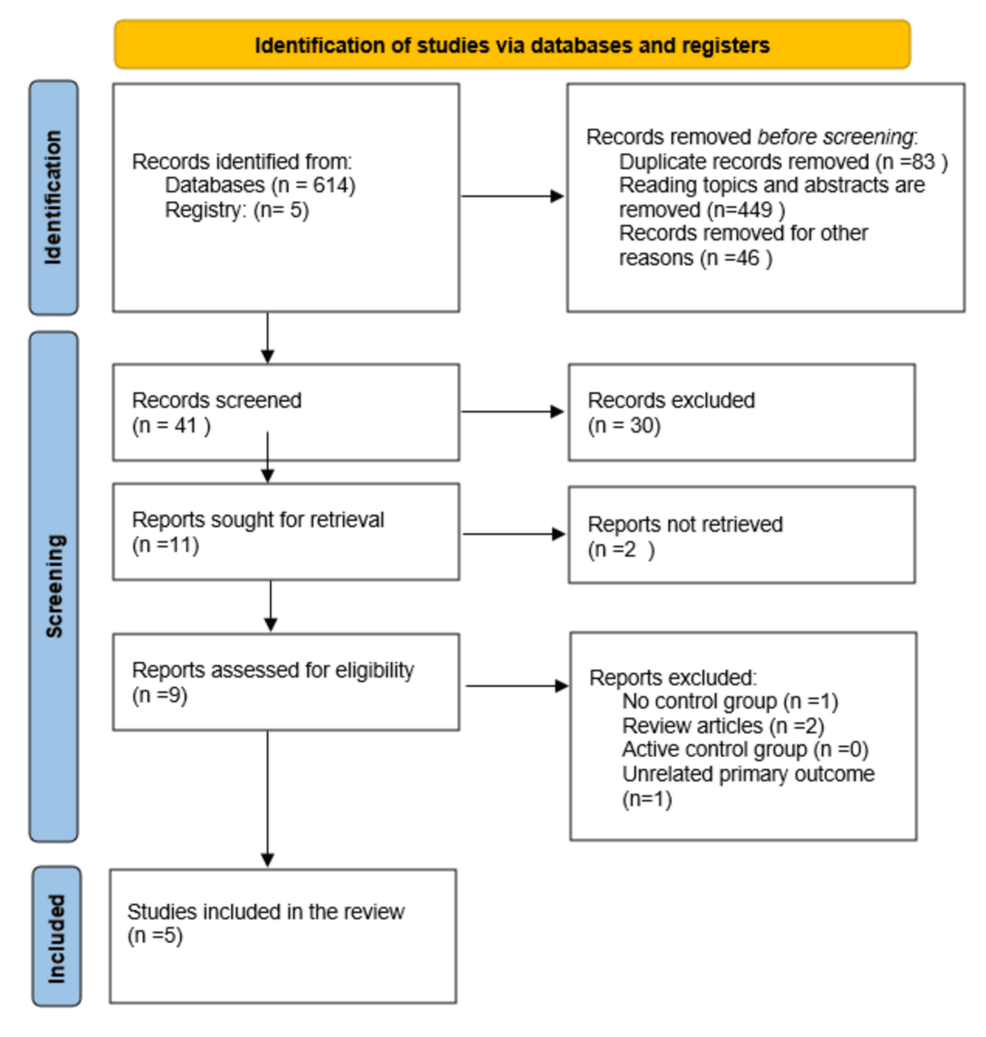
We identified 614 articles based on the inclusion criteria mentioned above. After removing duplicates and excluding articles that were not relevant, 41 titles were screened, out of which 30 were excluded. From the remaining 11 articles, 2 articles were not retrieved as they were not relevant. Of the remaining 9 articles, 4 were excluded (one with no control group, two review articles, and one with unrelated primary outcomes). Finally, 5 RCTs were selected for a qualitative systematic review and quantitative meta-analysis [19-23]. The PRISMA flow diagram is presented in Figure 1. Study characteristics and outcome details are summarized in Tables 2–3.
Qualitative Systematic Review
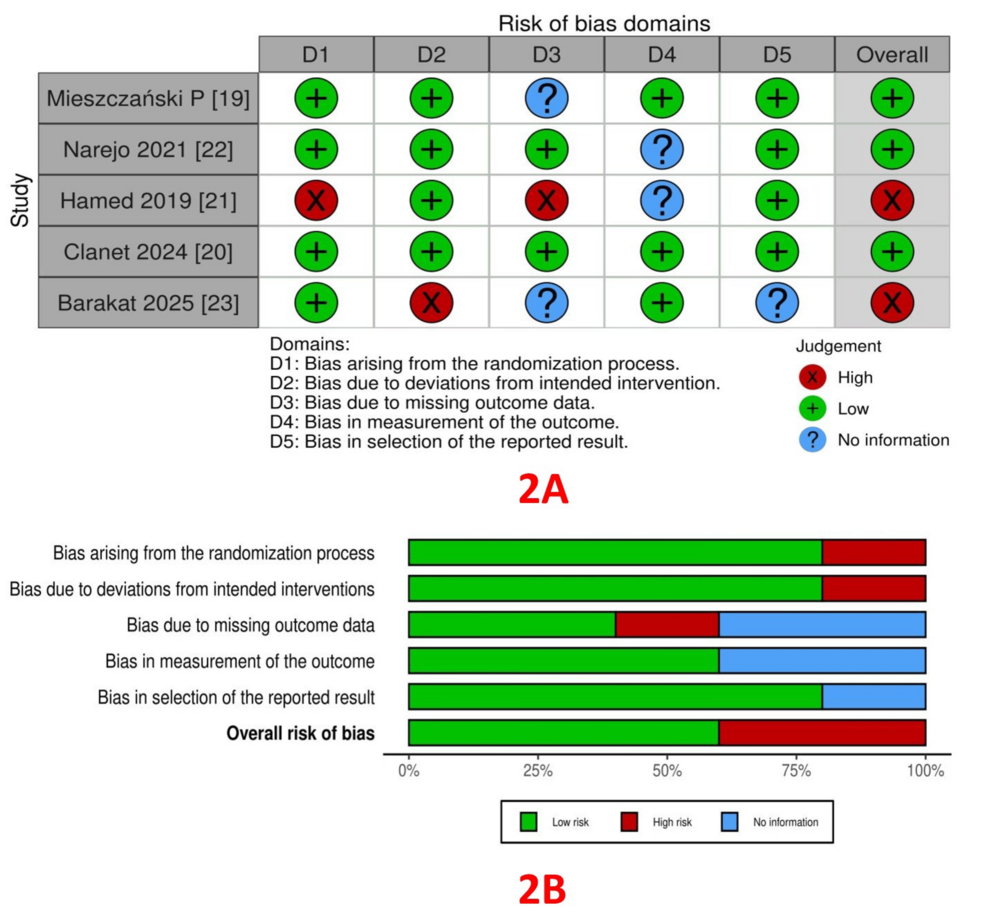
RoB assessment: The RoB within the trials, assessed using the ROB2 tool, is depicted in Figure 2A (traffic light plot) and Figure 2B (summary plot). Bias from the randomization process was low in 4 studies [19, 20, 22, 23] and high in one study [21]. Bias due to deviations from intended interventions (allocation concealment) was low in four studies [19-22] and high in one study [23]. Bias arising from missing outcome data was low in two studies [20, 22], high in one study [21], and not reported in two studies [19, 23]. Bias in outcome measurement was low in three studies [19, 20, 23], and not reported in two studies [21, 22]. Bias arising from the selection of reported results was low in four studies [19-22] and high in one study [23].
Quality of evidence: GRADE assessment was performed for three outcomes – 24-hour opioid consumption, pain scores, and PONV (Table 4). The level of evidence was low for 24-hour opioid consumption and PONV, and moderate for pain scores.
Quantitative Meta-Analysis
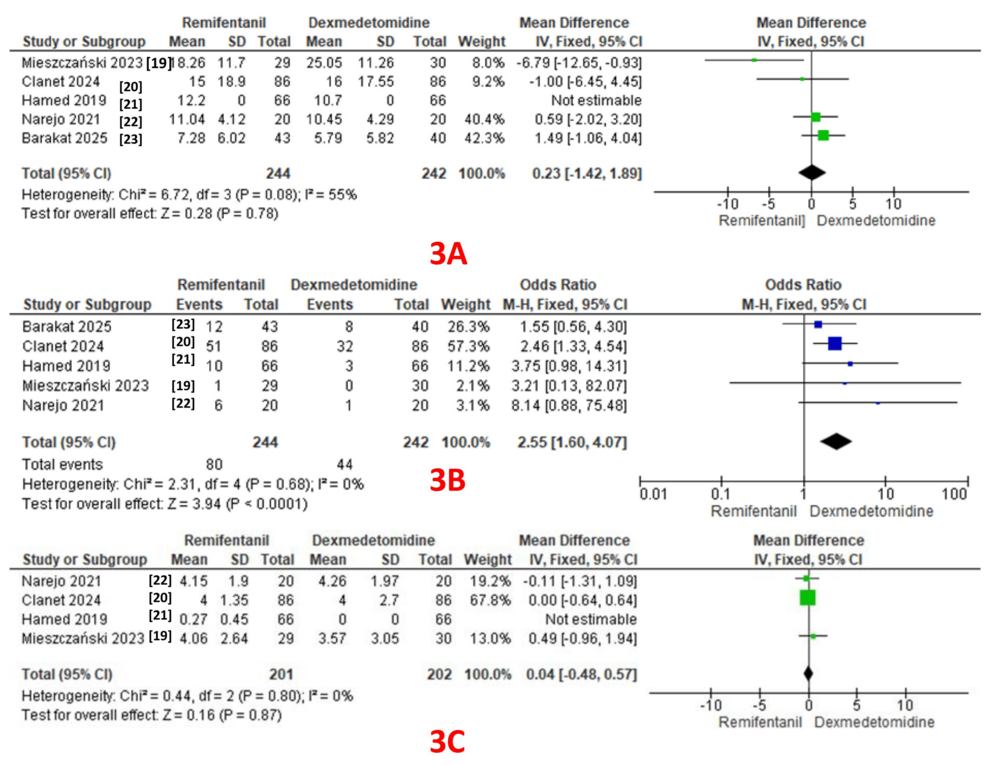
Twenty-four-hour opioid consumption was reported as an outcome in all five studies (244 patients in the remifentanil group and 242 patients in the dexmedetomidine group) [19-23]. A pooled analysis revealed comparable 24-hour opioid consumption in both groups (MD: 0.23; 95% CI: -1.42 to 1.89, P = 0.78). A fixed-effect model revealed moderate heterogeneity (I² = 55%) (GRADE = low) (Figure 3A).
PONV: PONV was reported as an outcome in all five studies (244 patients in the remifentanil group and 242 patients in the dexmedetomidine group), with 80 events in the remifentanil group and 44 in the dexmedetomidine group [19-23]. A pooled analysis revealed a significantly lower incidence of PONV in the dexmedetomidine group (OR: 2.55; 95% CI: 1.60 to 4.07, P < 0.0001). A fixed-effect model revealed no heterogeneity (I² = 0%) (GRADE = low) (Figure 3B).
Pain scores in the recovery room: Four studies reported pain scores as an outcome (201 patients in the remifentanil group and 202 patients in the dexmedetomidine group) [19-22]. A pooled analysis revealed comparable pain scores in both groups (MD: 0.04; 95% CI: -0.48 to 0.57, P = 0.87). A fixed-effect model revealed no heterogeneity (I² = 0%) (GRADE = moderate) (Figure 3C).
Surgery Duration
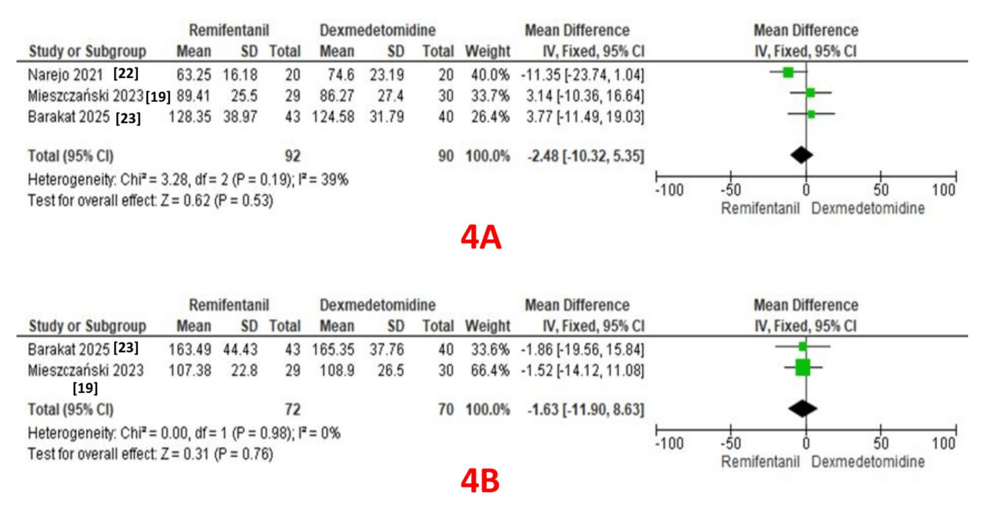
Three studies reported surgery duration as an outcome (92 patients in the remifentanil group and 90 patients in the dexmedetomidine group) [19, 22, 23]. A pooled analysis revealed comparable durations in both groups (MD: -2.48; 95% CI: -10.32 to 5.35, P = 0.53). A fixed-effect model revealed moderate heterogeneity (I² = 39%) (Figure 4A).
Two studies reported anesthesia duration as an outcome (72 patients in the remifentanil group and 70 patients in the dexmedetomidine group) [19, 23]. A pooled analysis revealed comparable durations in both groups (MD: -1.63; 95% CI: -11.0 to 8.63, P = 0.76). A fixed-effect model revealed no heterogeneity (I² = 0%) (Figure 4B).
TSA Findings
We performed TSA for only one outcome, PONV, as it demonstrated a significantly lower incidence in the dexmedetomidine group. TSA revealed that the information size (n = 231) reached 82.5% of the estimated RIS (n = 280), which was not substantially lower. As the cumulative Z-score curve crossed the conventional boundary or the trial sequential monitoring boundary, statistical significance was achieved (Figure 5).
Discussion
Summary of Results
This systematic review and meta-analysis compared the analgesic efficacy and safety of remifentanil with dexmedetomidine as adjuncts to general anesthesia in adult patients undergoing bariatric and metabolic surgeries. Notably, this is the first review to compare an opioid with a non-opioid adjunct in bariatric surgical patients. The overall RoB in the included studies was low, while the GRADE quality of evidence ranged from moderate to low. A pooled analysis revealed that analgesic efficacy, assessed by 24-hour opioid consumption and pain scores in the recovery room, was comparable between the two groups. However, the incidence of PONV was significantly lower in the dexmedetomidine group, a finding supported by the TSA, despite a lower RIS. The overall heterogeneity among the included studies for various outcomes was low. Utilizing only RCTs for the review was a strength of our work.
The intraoperative infusion of remifentanil has been successfully used in various surgeries. Its short-acting nature and rapid recovery profile make it beneficial for certain procedures [24]. However, some studies have raised concerns about rapid tolerance, opioid-induced hyperalgesia, and an increased need for rescue analgesia in the form of opioids, leading to higher rates of postoperative adverse events such as PONV, drowsiness, and constipation. In a quantitative review of 85 studies involving 13,057 patients, Komatsu R et al. concluded that, compared to other short-acting opioids (fentanyl, alfentanil, or sufentanil), remifentanil facilitated faster recovery after general anesthesia [25]. However, the incidence of PONV was comparable, and the incidence of shivering was twice as high as with other opioids. They also mentioned that remifentanil does not confer any additional advantages over other opioids in prolonged or major surgeries.
In a systematic review by Al-Hassan A et al., postoperative pain and opioid use were compared in patients who received either remifentanil or dexmedetomidine [26]. The authors concluded that while remifentanil led to rapid onset of analgesia and faster recovery, it also resulted in increased postoperative opioid requirements. On the contrary, dexmedetomidine was associated with slower recovery and prolonged extubation but resulted in lower postoperative pain scores with significant opioid-sparing effects. In another systematic review, the authors evaluated the safety and efficacy of remifentanil compared to other agents, including sufentanil and dexmedetomidine [27]. Owing to significant heterogeneity between the interventional and control groups, the authors concluded that the current evidence was equivocal about the intraoperative use of remifentanil in bariatric surgeries. Opioid-sparing and opioid-free anesthesia (OFA) are gaining popularity in current clinical practice and are increasingly encouraged across specialties [28,29]. Dexmedetomidine has emerged as a safe and effective opioid-sparing medication, offering perioperative benefits such as reduced PONV and fewer adverse effects commonly associated with opioids. In a systematic review, Wang G et al. concluded that intraoperative dexmedetomidine use significantly reduced PONV and pruritus compared to placebo [30]. Several reviews have also confirmed that, when used as an adjunct, dexmedetomidine improves pain scores, decreases opioid consumption, lowers the incidence of PONV, and reduces postoperative delirium [31,32]. A single-center experience with a meta-analysis by Chang PC et al. further supported these findings, concluding that perioperative dexmedetomidine improves postoperative pain control and reduces overall PONV compared to its absence [33].
Subramaniam T et al. conducted a systematic review investigating the efficacy of dexmedetomidine in reducing PONV in patients undergoing bariatric surgery [34]. Based on 13 RCTs, the authors concluded that dexmedetomidine was more effective as PONV prophylaxis when the duration of surgery was less than 120 minutes. Our review findings align with this conclusion. In another systematic review and meta-analysis, Xu N et al. compared remifentanil and dexmedetomidine for controlled hypotension in certain surgeries. While both agents provided comparable hypotensive effects, dexmedetomidine was associated with better pain scores, less pruritus, and lower PONV compared to remifentanil [35]. However, the time to extubation was shorter in the remifentanil group. In a retrospective study of 134 patients undergoing bariatric surgery, Nam SW et al. compared recovery characteristics between those who received intraoperative remifentanil or dexmedetomidine [36]. They concluded that dexmedetomidine was associated with better pain scores, lower PONV, and reduced analgesic requirements in the immediate postoperative period. Singh PM et al. performed a meta-analysis with trial sequential analysis to investigate the perioperative analgesic profile of dexmedetomidine infusions in morbidly obese patients undergoing bariatric surgery [37]. Their analysis included six studies comparing dexmedetomidine with either an opioid (fentanyl or morphine) or placebo (saline). The results showed that patients in the dexmedetomidine group had lower opioid consumption during both the immediate and extended recovery phases, better pain control, and lower PONV. In a clinical study, Clanet M et al. compared a multimodal OFA technique to a multimodal opioid-based strategy during bariatric surgery [20]. They found no significant difference in postoperative morphine consumption between the groups. While the overall quality of recovery was similar, the OFA group experienced a lower incidence of PONV.
The strength of this review is that all included studies in the analysis were RCTs. However, some had small sample sizes, which could have influenced the findings. Despite this, most RCTs demonstrated an overall low RoB, further strengthening this review. Additionally, the TSA demonstrated statistical significance for PONV, confirming that dexmedetomidine was superior in reducing its incidence, although the RIS was 82.5%, which is not very low. Several important outcomes, such as length of stay (recovery room, hospital), patient satisfaction, and cost efficiency, were either inconsistently reported or not reported at all, preventing their analysis. Further studies should address these factors to provide clinicians with a more comprehensive comparison of medications.
Conclusions
Based on the findings of this systematic review and meta-analysis, we conclude that remifentanil and dexmedetomidine offer comparable analgesic efficacy, as reflected by 24-hour opioid consumption and immediate postoperative pain scores, when used as adjuncts to general anesthesia in bariatric and metabolic surgeries. Dexmedetomidine was found to be associated with a significantly lower incidence of PONV compared to remifentanil. However, the current body of evidence is of moderate to low certainty. Further well-designed and adequately powered studies are warranted to determine the optimal anesthetic adjunct in this patient population.
References
- Dority J, Hassan ZU, Chau D: Anesthetic implications of obesity in the surgical patient. Clin Colon Rectal Surg. 2011, 24:222-228. 10.1055/s-0031-1295685
- Kaye AD, Lingle BD, Brothers JC, Rodriguez JR, Morris AG, Greeson EM, Cornett EM: The patient with obesity and super-super obesity: perioperative anesthetic considerations. Saudi J Anaesth. 2022, 16:332-338. 10.4103/sja.sja_235_22
- Hardt K, Wappler F: Anesthesia for morbidly obese patients. Dtsch Arztebl Int. 2023, 120:779-785. 10.3238/arztebl.m2023.0216
- Naaz S, Ozair E: Dexmedetomidine in current anaesthesia practice-a review. J Clin Diagn Res. 2014, 8:GE01-GE04. 10.7860/JCDR/2014/9624.4946
- Piao G, Wu J: Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci. 2014, 10:19-24. 10.5114/aoms.2014.40730
- Cohen J, Royston D: Remifentanil. Curr Opin Crit Care. 2001, 7:227-231. 10.1097/00075198-200108000-00003
- Beers R, Camporesi E: Remifentanil update: clinical science and utility. CNS Drugs. 2004, 18:1085-1104. 10.2165/00023210-200418150-00004
- Greenwood J: Anesthetic implications of obesity and obstructive sleep apnea. Annu Rev Nurs Res. 2017, 35:17-35. 10.1891/0739-6686.35.17
- Altamimi R, Alnajjar D, Bin Salamah R, et al.: Dexmedetomidine in bariatric surgery: a systematic review and meta-analysis of its effects on postoperative pain and postoperative nausea and vomiting. J Clin Med. 2025, 14:679. 10.3390/jcm14030679
- Silva LM, Silveira SQ, Abib AD, Nunes WP, Mittermayer O, Oliveira DR: Comparative analysis of remifentanil versus dexmedetomidine in the incidence of pain in a post-anesthesia care unit after bariatric surgery. BrJP. 2018, 1:217-222. 10.5935/2595-0118.20180043
- Sterne JA, Savović J, Page MJ, et al.: RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019, 366:l4898. 10.1136/bmj.l4898
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (Developed by Evidence Prime, Inc.). (2025). Accessed: March 17, 2025: https://www.gradepro.org.
- Review Manager (RevMan) [Computer Program]. Version 5.4.; The Cochrane Collaboration. (2020). Accessed: March 17, 2025: http://revman.cochrane.org.
- Stogiannis D, Siannis F, Androulakis E: Heterogeneity in meta-analysis: a comprehensive overview. Int J Biostat. 2024, 20:169-199. 10.1515/ijb-2022-0070
- Dettori JR, Norvell DC, Chapman JR: Fixed-effect vs random-effects models for meta-analysis: 3 points to consider. Global Spine J. 2022, 12:1624-1626. 10.1177/21925682221110527
- Nair AS, Borkar N: Sensitivity and subgroup analysis in a meta-analysis – What we should know?. Indian J Anaesth. 2024, 68:922-924. 10.4103/ija.ija_623_24
- Sterne JA, Egger M: Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001, 54:1046-1055. 10.1016/s0895-4356(01)00377-8
- Kang H: Trial sequential analysis: novel approach for meta-analysis. Anesth Pain Med (Seoul). 2021, 16:138-150. 10.17085/apm.21038
- Mieszczański P, Górniewski G, Ziemiański P, Cylke R, Lisik W, Trzebicki J: Comparison between multimodal and intraoperative opioid free anesthesia for laparoscopic sleeve gastrectomy: a prospective, randomized study. Sci Rep. 2023, 13:12677. 10.1038/s41598-023-39856-2
- Clanet M, Touihri K, El Haddad C, et al.: Effect of opioid-free versus opioid-based strategies during multimodal anaesthesia on postoperative morphine consumption after bariatric surgery: a randomised double-blind clinical trial. BJA Open. 2024, 9:100263. 10.1016/j.bjao.2024.100263
- Hamed JM, Refaat HS, Al-Wadaani H: Dexmedetomidine compared to remifentanil infusion as adjuvant to sevoflurane anesthesia during laparoscopic sleeve gastrectomy. Anesth Essays Res. 2019, 13:636-642. 10.4103/aer.AER_126_19
- Narejo AS, Khan MM, Alwhabi A, Alqarni A, Ahmed AE, Eldawlatly AA: Impact of intraoperative dexmedetomidine versus remifentanil on recovery characteristics following laparoscopic sleeve gastrectomy. J Coll Physicians Surg Pak. 2021, 31:210-214. 10.29271/jcpsp.2021.02.210
- Barakat H, Gholmieh L, Nader JA, Karam VY, Albaini O, Helou ME, Al Nawwar R: Opioid-free versus opioid-based anesthesia in laparoscopic sleeve gastrectomy: a single-center, randomized, controlled trial. Perioper Med (Lond). 2025, 14:16. 10.1186/s13741-024-00486-5
- Sivak EL, Davis PJ: Review of the efficacy and safety of remifentanil for the prevention and treatment of pain during and after procedures and surgery. Local Reg Anesth. 2010, 3:35-43. 10.2147/lra.s7709
- Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC: Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007, 62:1266-1280. 10.1111/j.1365-2044.2007.05221.x
- Al-Hassan A, Weissman B, Chowdhury S, Sawires J, Soti V: Comparative efficacy of dexmedetomidine and remifentanil in reducing postoperative pain and opioid use: a systematic review. Cureus. 2025, 17:e79759. 10.7759/cureus.79759
- Nair AS, Gurajala I, Borkar N, Dudhedia U, Rangaiah M, Diwan S: Safety and efficacy of remifentanil in patients undergoing bariatric and metabolic surgeries – a systematic review. Indian J Anaesth. 2025, 69:123-131. 10.4103/ija.ija_825_24
- Sakan S, Turudić Ž, Peremin S, et al.: Opioid free general anesthesia in clinical practice – a review article. Acta Clin Croat. 2023, 62:362-367. 10.20471/acc.2023.62.02.15
- Lauretta MP, Marino L, Bilotta F: Safety and efficacy of opioid-sparing anesthesia compared with traditional opioid anesthesia: a scoping review. Clin J Pain. 2025, 41:1261. 10.1097/AJP.0000000000001261
- Wang G, Zhang L, Lou S, et al.: Effect of dexmedetomidine in preventing postoperative side effects for laparoscopic surgery: a meta-analysis of randomized controlled trials and trial sequential analysis (PRISMA). Medicine (Baltimore). 2016, 95:e2927. 10.1097/MD.0000000000002927
- Zhang X, Leng Y, Yuan X, Yang Y, Zhou C, Liu H: Efficacy of perioperative dexmedetomidine in postoperative pain and neurocognitive functions in orthopedic surgery: a systematic review and meta-analysis with trial sequential analysis of randomized controlled trials. Int J Surg. 2025, 111:3525-3542. 10.1097/JS9.0000000000002315
- Liu Y, Zhao G, Zang X, Lu F, Liu P, Chen W: Effect of dexmedetomidine on opioid consumption and pain control after laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. Wideochir Inne Tech Maloinwazyjne. 2021, 16:491-500. 10.5114/wiitm.2021
- Chang PC, Huang IY, Liu SD, et al.: Perioperative dexmedetomidine infusion improves perioperative care of bariatric-metabolic surgery: a single center experience with meta-analysis. Obes Surg. 2024, 34:416-428. 10.1007/s11695-023-07036-w
- Subramaniam T, Tan HY, Tan JH, Pung JW, Htet H: Efficacy of dexmedetomidine in postoperative nausea and vomiting in laparoscopic bariatric surgery: a systematic review and meta-analysis of randomised clinical trials. Med J Malaysia. 2024, 79:626-645.
- Xu N, Chen L, Liu L, Rong W: Dexmedetomidine versus remifentanil for controlled hypotension under general anesthesia: a systematic review and meta-analysis. PLoS One. 2023, 18:e0278846. 10.1371/journal.pone.0278846
- Nam SW, Oh AY, Koo BW, Kim BY, Han J, Yoon J: Effect of dexmedetomidine compared to remifentanil during bariatric surgery on postoperative nausea and vomiting: a retrospective study. Obes Surg. 2022, 32:3368-3374. 10.1007/s11695-022-05894-4
- Singh PM, Panwar R, Borle A, Mulier JP, Sinha A, Goudra B: Perioperative analgesic profile of dexmedetomidine infusions in morbidly obese undergoing bariatric surgery: a meta-analysis and trial sequential analysis. Surg Obes Relat Dis. 2017, 13:1434-1446. 10.1016/j.soard.2017.02.025