Intrahospital transport is a common occurrence for many hospitalized patients. Critically ill children are an especially vulnerable population who experience preventable adverse events at least once a week, on average.1 Transporting these patients throughout the hospital introduces additional hazards and increases the risk of adverse events.2 The transport process can be decomposed into a series of steps, each incurring specific risk. These risks are numerous and few of these risks are specific to the transport process. There is a paucity of literature available on pediatric intrahospital transport and related adverse events. Therefore, we recently reviewed the Wake Up Safe database, a pediatric anesthesia quality improvement initiative across member institutions to disseminate information on best practices, for pediatric perioperative adverse events associated with anesthesia-directed transport. Below we present several examples of airway and respiratory events taken from the database and discuss the complexity of the transport process.
AIRWAY AND VENTILATION MANAGEMENT CASE VIGNETTES
Case #1: 2-week-old, former 32-week premature infant underwent largely uneventful exploratory laparotomy in the operating room (OR) for presumed necrotizing enterocolitis. On arrival to the intensive care unit (ICU), the infant was transitioned to the ventilator with assistance from the respiratory therapist. The ventilator tubing fell, dislodging the endotracheal tube (ETT). The patient rapidly deteriorated requiring chest compressions and reintubation. After several minutes of cardiopulmonary resuscitation (CPR), return of spontaneous circulation was achieved and the patient stabilized over the next several hours.
Case #2: 8-month-old infant with complex medical history including congenital hydrocephalus status post ventriculoperitoneal shunt placement, recurrent pneumonia, and current respiratory failure was scheduled for tracheostomy placement. Patient was transported to the operating room with an ETT in situ. Following transfer from patient stretcher to the OR table, the team also converted the patient from a state of spontaneous ventilation with a Jackson-Rees circuit to mechanical ventilation. Within one minute of this transition, the patient became difficult to ventilate, acutely hypoxemic, and subsequently asystolic. CPR was initiated and a repeat laryngoscopy was performed due to concern for ETT dislodgement. The ETT was replaced and shortly thereafter, there was restoration of normal sinus rhythm. The post-event review diagnosed bronchospasm and noted that a routine morning chest x-ray from that day showed the ETT positioned in the right bronchus. This was not reviewed by the anesthesia team prior to transport, in part due to task overload.
Case #3: Ventilatory changes after sedation and neuromuscular blockade: 11-month-old infant in the ICU, ETT in situ, who required reoperation for bleeding following Tetralogy of Fallot repair earlier in the day. In preparation for transport to the operating room, the team administered midazolam and rocuronium. Shortly after medication administration, the patient became difficult to hand ventilate. The patient quickly became hypoxic, followed by pulseless electrical activity. CPR was initiated and during resuscitation, a large mucus plug was suctioned from the ETT. Afterwards ventilation improved significantly and return of spontaneous circulation was achieved. The remainder of the procedure and the perioperative transport was without further incident.
AIRWAY AND VENTILATION MANAGEMENT RISKS
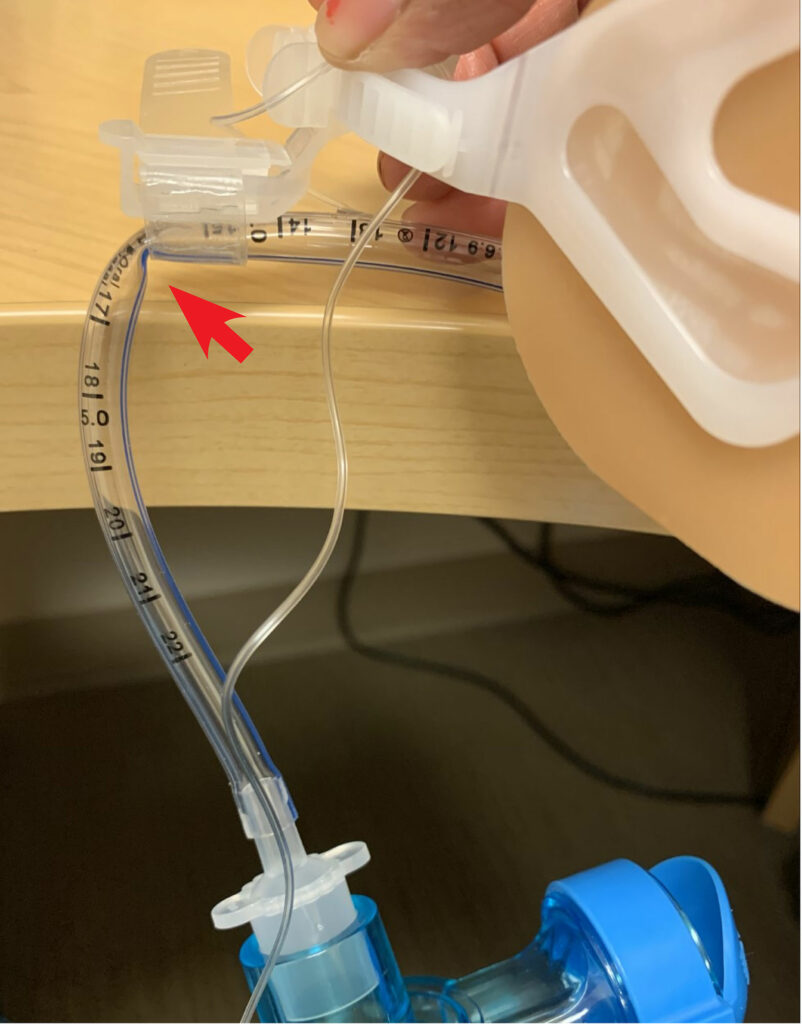
Figure 1a: Endotracheal tubes secured with Hollister (Hollister Inc., Libertyville, IL) endotracheal tube fastener, with kink when attached to Ambu bag (Ambu Inc., Columbia, MD) without offloading the weight of the circuit/ventilation system.
The majority of complications in the transport of critically-ill and anesthetized pediatric patients are respiratory in nature.3 From the Wake Up Safe data, approximately 40% of transport-related events occurred in patients less than or equal to 6 months of age, and a large majority occurred in patients with American Society of Anesthesiologists (ASA) 3 status or greater.3 Of the 15 unplanned extubations reported, 14 occurred in patients less than or equal to 6 months of age and 11 of 15 occurred in patients less than 4 kg. One reason for the higher rate of unintended extubation is the practice of positioning the ETT between the first and second thoracic vertebrae in the neonatal ICU, which reduces nonuniform lung aeration, localized pulmonary interstitial emphysema, and pneumothorax.4 However, this position may increase risk for inadvertent extubation if there is extension of the head/neck that may lead to cephalad movement of the ETT.5,6 On the contrary, ETTs that are positioned close to the carina can lead to mainstem intubation with inadvertent caudad movement, leading to hypoxemia, hypercarbia, pneumothorax, and mucosal injury.4,7 Therefore, we recommend review of the most recent chest x- ray and positioning the ETT in the mid-thoracic trachea for transportation to mitigate this risk. Auscultation of bilateral breath sounds and utilization of continuous capnography can also mitigate these risks. A pillow can be used to help stabilize the head and care taken to avoid any tension on the ETT during transport. During transportation, the removal of these ventilator circuit holders that off-load tension while in the ICU can lead to ETT obstruction from kinking of smaller ETTs (Figure 1a and Figure 1b). Caution should be taken to ensure the ETT and circuit are positioned in a way to prevent kinking by off-loading the weight of breathing circuits used during transportation. A transport ventilator provides more consistent minute ventilation and will avoid hypo- or hypercarbia in high-risk patients.8,9 However, it will not prevent the risks associated with inappropriate ETT positioning, kinking, or obstruction. The specific devices that secure ETT to the face can vary from unit and institution, but typically securing devices that minimize skin breakdown are preferred in pediatric patients in the ICU. Furthermore, the seemingly simple act of moving an intubated patient can be quite stimulating, which can result in sympathetic activation, leading to tachycardia, agitation, and coughing, which may lead to bronchospasm from airway irritability. Movement may result in altered pulmonary compliance and the ability to provide adequate oxygenation and ventilation.

Figure 1b: Endotracheal tubes secured with NeoBar ET tube (NeoTech Products LLC, Valencia, CA) with kink when attached to ambu bag (Ambu Inc., Columbia, MD) without offloading the weight of the circuit/ventilation system.
Invasive ventilation is a risk factor for mucus plugging given impaired mucociliary clearance10; add to that sedative or neuromuscular blocking agents and the intrinsic ability to cough and expel mucus is further impaired. During transportation, patients are typically transported without heat and humidification of airway gases which can perpetuate mucus plug formation. Many clinicians elect to administer neuromuscular blockade along with sedation medication to intubated patients. The benefits of giving neuromuscular blockade for transport include eliminating ventilator dyssynchrony, which can be obviated by using a modern portable ventilator. Neuromuscular blockade can reduce the risk of unplanned line or tube removal in agitated patients and also reduce transport team workload. There are also potential unintended consequences when using neuromuscular blockade for transportation of intubated pediatrics patients. It has been associated with worsened mucus plugging of the endotracheal tube leading to two cardiac arrests in two children through unclear mechanisms.3,11 It eliminates patient respiratory effort, which may require changes in ventilator settings and can worsen an existing endotracheal tube leak. Additionally, sedative medications may reduce sympathetic tone creating the potential for hypotension, and neuromuscular blockade may reduce basal metabolism which may lead to hypocarbia. The decision to use neuromuscular blocking agents and sedatives during the transportation of pediatric patients should be predicated on the aforementioned advantages and disadvantages.
IDENTIFYING AND MITIGATING RISK
Before any transport of a critically ill child is undertaken, the risks, benefits, and alternatives should be carefully considered. Potential for harm includes line dislodgement, derangements in hemodynamics, unplanned extubation, hypoxemia, hypo- and hypercarbia, hemorrhage, pneumothorax, elevation of ICP in at-risk patients, hypothermia, and increased risk for hospital-acquired infections.3,12-15 If a patient is on an advanced mode of ventilation, such as high-frequency oscillatory or jet ventilation (HFOV/HFJV), or on an extra-corporeal device, such as extra-corporeal membrane oxygenation (ECMO), there should be a multidisciplinary discussion regarding the risk of transportation to the radiology, procedural, or operating room suites versus having their diagnostic or therapeutic procedure done at the bedside. Whenever feasible, bedside alternatives should be strongly considered for high-risk patients.
Postoperative transport appears to be a period associated with numerous potential complications. Almost 75% of respiratory complications and 70% of cardiac arrests occurred in the postoperative period.3 For patients who received anesthetics, patients may emerge from anesthesia during transport. Many patients are extubated prior to postoperative transport, during which it is often more difficult to detect or treat respiratory adverse events. This is due to the increased cognitive load of navigating hallways, availability of emergency equipment, and assistance. In fact, task overload was often noted as a secondary contributor in these events.3
EFFECTIVE COMMUNICATION AND TEAMWORK
We recommend the use of standardized handover tools, appropriate training of providers directly involved in transportation, and close communication with ordering clinicians regarding the possible risks associated with transporting patients throughout the hospital. Freely available and validated tools are available here: https://www.handoffs.org/patient-handoff-resources/. Each team member involved in transportation should have a specific role, with a dedicated provider for airway management, medication administration and maneuvering the bed and other devices, as needed. It may be “just another imaging study” to facilitate a diagnosis or a simple procedure to progress care, but if not weighed carefully, it could lead to serious and catastrophic complications for patients, families, clinicians, ancillary staff, and even visitors. Whenever possible, consideration should be given to available bedside alternatives.
Checklists to ensure that all pertinent information is transferred correctly as well as confirming necessary equipment and emergency medications are available, may help this sometimes overwhelming task seem more manageable and prevent information from being lost. Bedside nurse handover for direct patient care report noting frequency of interventions such as fluid/medication boluses or infusion changes or ETT suction frequency may provide context for changes in patient status.
Critical events are best managed by a team with a clear leader, effective communication, and clear roles for team members.16 These principles have been applied to cardiac arrest, life support, trauma, and during complex resuscitations in the operating room. These principles can also be applied to the transport of critically ill and anesthetized children. A team leader should be clearly identified, and for unstable or complex patients, they should have no other tasks other than leading the team. Ensuring there are the appropriate number of skilled team members dedicated to every task during transportation is important. The bed may be pushed by ancillary staff so the medical and nursing teams can focus on the care of the patient. Patients who rely on physiologic support such as a ventilator, vasoactive infusion, or mechanical circulatory support require dedicated and appropriately skilled staff for each task. Patients who require frequent sedative, vasopressor, or hypertonic saline boluses may require a provider be dedicated solely to these tasks during transport.
CULTURE OF SAFETY
Creation of local standardized processes for transport and team training should improve the culture of safety around transport. There is no national or international standard of care for the intrahospital transportation of patients, and there are limited data to validate a specific transport team at this time. As described above, careful risk assessment is essential. Patients with reliance on lifesaving technologies such as mechanical ventilation, vasoactive medications or a ventriculostomy will require a transport team knowledgeable, skilled, and experienced at using those technologies, with appropriate backup equipment and medications. Two studies identified junior trainee physicians to have experienced higher rates of adverse events than senior trainees/faculty.17,18 When possible, a senior member of the team should transport with critically ill patients and help train junior clinicians. A recent multicenter study showed that a positive safety climate and effective team processes were associated with fewer adverse events during intrahospital transport of critically-ill adults.19 Team experience and mandatory training also reduced adverse events.19
CONCLUSIONS
Intrahospital transport represents the intersection of numerous patient safety concerns—airway management, early recognition of clinical deterioration, communication, and teamwork.20 In our recent review of pediatric intrahospital transport events in the Wake Up Safe database, the populations most at risk were those less than or equal to 6 months of age and children with more severe medical comorbidities. Despite the relatively short time that intrahospital transport requires, this phase of care may represent up to 5% of all pediatric anesthesia adverse events.3 Standardized risk assessment and resource allocation and structured handovers are an essential way to begin to improve our care during this potentially tumultuous period.
REFERENCES
- Larsen GY, Donaldson AE, Parker HB, Grant MJC. Preventable harm occurring to critically ill children. Pediatr Crit Care Med. 2007;8:331–336. PMID: 17417126
- Bergman LM, Pettersson ME, Chaboyer WP, et al. Safety hazards during intrahospital transport: a prospective observational study. Crit Care Med. 2017;45:e1043–e1049. PMID: 28787292
- Haydar B, Baetzel A, Stewart M, et al. Complications associated with the anesthesia transport of pediatric patients: an analysis of the Wake Up Safe database. Anesth Analg. 2020;131:245–254. PMID: 31569160
- Thayyil S, Nagakumar P, Gowers H, Sinha A. Optimal endotracheal tube tip position in extremely premature infants. Am J Perinatol. 2008;25:13–17. PMID: 18027311
- Phipps L, Thomas N., Gilmore R, et al. Prospective assessment of guidelines for determining appropriate depth of endotracheal tube placement in children. Pediatr Crit Care Med. 2005;6:519–522. PMID: 16148809
- Sugiyama K, Yokoyama K. Displacement of the endotracheal tube caused by change of head position in pediatric anesthesia: evaluation by fiberoptic bronchoscopy. Anesth Analg. 1996;82:251–253. PMID: 8561322
- Zuckerberg, AL, Nichols, DG: Airway management in pediatric critical care. In: Textbook of Pediatric Intensive Care. Third Edition. Rogers MC (Ed). Baltimore, Williams and Wilens, 1996.
- Bachiller PR, McDonough JM, Feldman JM. Do new anesthesia ventilators deliver small tidal volumes accurately during volume-controlled ventilation? Anesth Analg. 2008;106:1392–1400. PMID: 18420850
- King MR, Feldman JM. Optimal management of apparatus dead space in the anesthetized infant. Paediatr Anaesth. 2017;27:1185–92. PMID: 29044830
- Wallen E, Venkataraman ST, Grosso MJ, et al. Intrahospital transport of critically ill pediatric patients. Crit Care Med. 1995;23:1588–1595. PMID: 7664562
- Murphy GS, Szokol JS, Marymont JH, et al. Intraoperative neuromuscular blockade and postoperative apnea in at-risk patients. Anesth Analg. 2009;108: 1338–1345. PMID: 32421054
- Konrad F, Schreiber T, Brecht-Kraus D, Georgieff M. Mucociliary transport in ICU patients. Chest. 1994; 105:237–241. PMID: 8275739
- Agrawal S, Hulme SL, Hayward R, Brierley J. A portable ct scanner in the pediatric intensive care unit decreases transfer-associated adverse events and staff disruption. Eur J Trauma Emerg Surg. 2010;36:346–352. PMID: 26816039
- Bastug O, Gunes T, Korkmaz L, et al. An evaluation of intra-hospital transport outcomes from tertiary neonatal intensive care unit. J Matern Fetal Neonatal Med. 2016;29:1993–1998. PMID: 26335382
- Marx G, Vangerow B, Hecker H, et al. Predictors of respiratory function deterioration after transfer of critically ill patients. Intensive Care Med. 1998;24:1157–1162. PMID: 9876978
- Rosen, M, DiazGranados D, Dietz, AS, et al. Teamwork in healthcare: key discoveries enabling safer, higher quality care. Am Psychol. 2018;73: 433–450. PMID: 29792459
- Harish MM, Siddiqui SS, Prabu NR, et al. Benefits of and untoward events during intrahospital transport of pediatric intensive care unit patients. Indian J Crit Care Med. 2017;21:46–48. PMID: 28197051
- Papson JP, Russell KL, Taylor DM. Unexpected events during the intrahospital transport of critically ill patients. Acad Emerg Med. 2007;14:574–577. PMID: 17535981
- Latzke M, Schiffinger M, Zellhofer D, Steyrer J. Soft factors, smooth transport? The role of safety climate and team processes in reducing adverse events during intrahospital transport in intensive care. Health Care Manage Rev. 2020; 45:32–40. PMID: 29176495
- Greenberg S. The APSF revisits its top 10 patient safety priorities. APSF Newsletter. 2021;36:1. https://www.apsf.org/article/the-apsf-revisits-its-top-10-patient-safety-priorities/. Accessed March 17, 2023.


Leave a Reply
You must be logged in to post a comment.