More than a decade ago the APSF established a clear edict: “No patient should be harmed by opioid-induced respiratory depression in the postoperative period.”1 Research studies established a strong association between obstructive sleep apnea (OSA) and adverse postoperative opioid-related outcomes. In response, medical societies issued perioperative guidelines calling for universal screening for OSA, continuation of OSA therapies in the postoperative period, and calls for the anesthesia team to appropriately modify the anesthetic and postoperative monitoring of patients.2,3 Unfortunately, the published rates of severe postoperative opioid-related respiratory depression (OIRD) have remained relatively constant.4
More recent studies have expanded our understanding of which patients are at the highest risk for severe OIRD. These results suggest we need a more wholistic approach of assessing patients beyond screening for OSA and begin to consider patient, surgical, anesthetic, and importantly anesthetic recovery characteristics. Also, these recent studies give us a better idea of when and how postoperative OIRD presents, allowing us to develop better postoperative monitoring strategies.
PATIENT CHARACTERISTICS
The association between severe OIRD and OSA is well established. For example, Mayo Clinic researchers have studied the administration of naloxone on postoperative wards as a proxy measure for severe OIRD.5,6 These studies found that patients with a history or positive screen for OSA have double the risk for developing severe postoperative OIRD compared to patients without OSA.5,6
These Mayo Clinic naloxone studies5,6 and the PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) trial7 have identified other important patient characteristics in addition to OSA, which also increase OIRD risk. The PRODIGY trial used bedside capnography and pulse oximetry on general care wards to identify episodes of OIRD (Figure 1). The PRODIGY researchers were then able to look at 46 potential patient risk factors to develop a risk score for OIRD (PRODIGY score, Table 1). While, as expected, OSA and other sleep breathing disorders were found to increase risk, so was older age, male sex, congestive heart failure, and opioid-naïvety; with age beyond 70 years being most important.7
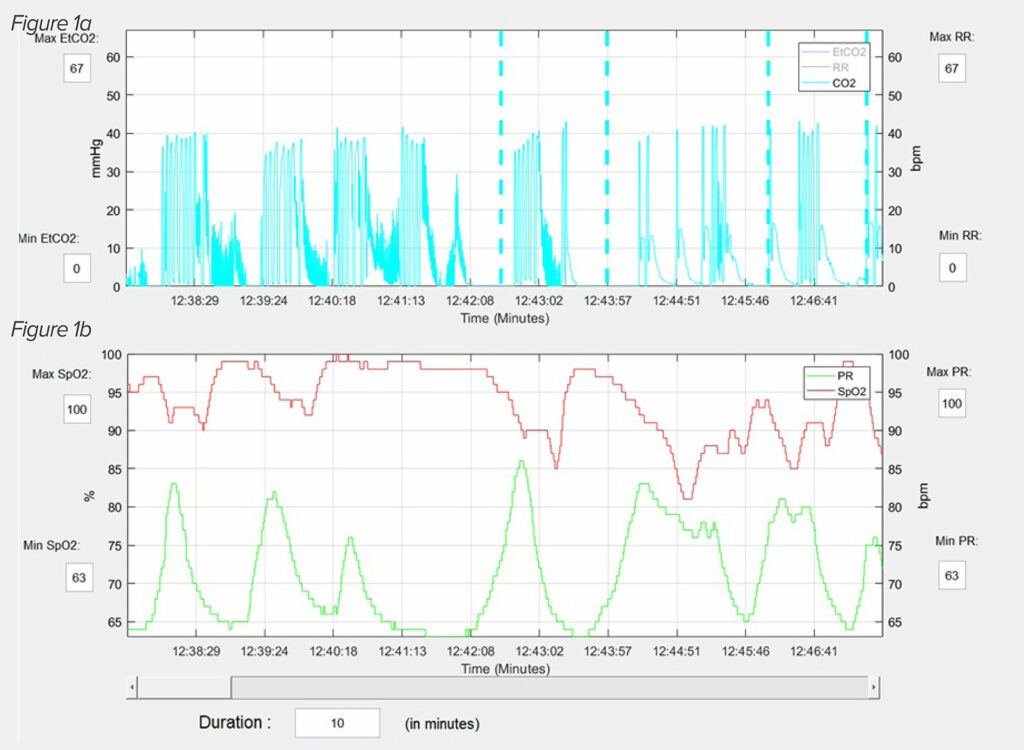
Figure 1: An actual capnography (1a) and pulse oximetry (1b) reading from PRODIGY, illustrates the typical OIRD breathing pattern.20 This patient is having repetitive apnea and partial apnea episodes interceded with normal breathing patterns. The periods of hypoxemia develop during the apnea episodes and the oxygen saturation normalizes when normal breathing resumes. Reprinted and modified with permission from Anesthesia & Analgesia and Wolters Kluwer Health, Inc.
Table 1: PRODIGY Scoring System for Assessing Risk for OIRD Among Patients Hospitalized on General Care Wards Receiving Opioids
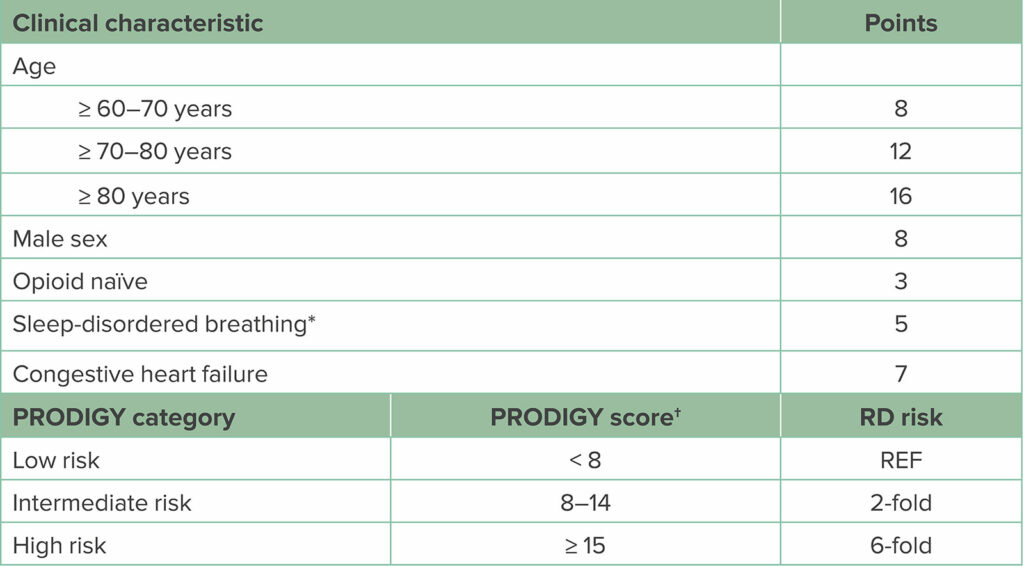
Abbreviations: PRODIGY, PRediction of Opioid-induced respiratory Depression in patients monitored by capnoGraphY; RD, respiratory depression; REF, reference range.
*Sleep-disordered breathing can be determined from either patient history or positive screen for sleep apnea.
†To calculate the PRODIGY risk score, summate the assigned points per positive clinical characteristic. Patients are assigned low-, intermediate-, or high-risk category based on the number of points. Compared to low-risk scored patients, intermediate-risk patients have a 2-fold increase and high-risk patients a 6-fold increased risk for experiencing respiratory depressive episodes on the general care ward. (Adapted from Khanna et al.7)
One weakness of PRODIGY was that many of these 46 factors were specific diagnoses and some were too rare (amyotrophic lateral sclerosis) to adequately examine. Instead, the Mayo Clinic naloxone studies5,6 used organ system disease to assess risk, and found cardiovascular disease, OSA, and debility more than doubled the OIRD risk, but that central neurologic diseases quadrupled OIRD risk. These studies suggest we should, in addition to OSA, also consider increasing age, disease burden, and debility as risk factors for OIRD.
PERIOPERATIVE COURSE
We should not just focus on patient factors when assessing OIRD risk, but also consider the perioperative course. More extensive and invasive procedures increase the risk for respiratory failure, while regional anesthetics may decrease risk.8 Different anesthetic drugs can increase or decrease the risk for OIRD while patients are admitted to the postanesthesia care unit (PACU). The Mayo Clinic has developed a unique protocol to manage patients in the PACU who are experiencing respiratory depression.9 In that protocol, OSA risk is assessed preoperatively and postoperatively. PACU nurses continuously monitor patients for episodes of respiratory depression (apnea, bradypnea, oxyhemoglobin desaturation, or “pain-sedation” mismatch (defined as when a heavily sedated patient complains of severe pain). Any patient who has one of these respiratory depressive episodes then undergoes monitoring for two additional 30-minute periods for additional episodes of respiratory depression. Those patients who have additional episodes of respiratory depression then undergo postoperative continuous monitoring with telemetry and are also considered for non-invasive positive pressure ventilation.9
Use of the soluble volatile anesthetic isoflurane, preoperative sustained release oxycodone administration, increasing doses of intraoperative opioids, and preoperative gabapentin were all found to increase PACU respiratory depression.10,11 When one clinical area at the Mayo Clinic substituted desflurane for isoflurane and avoided routine use of midazolam, episodes of PACU respiratory depression decreased by 30%.12
Gabapentin and pregabalin continue to increase the risk for OIRD after PACU discharge. One study found that patients using gabapentin at home who then continued gabapentin postoperatively were at a 6-fold increase risk for naloxone administration.5 Researchers using the Premier Healthcare Database found that the use of preoperative gabapentin and pregabalin (as part of an Enhanced Recovery After Surgery [ERAS] multimodal protocol) increased the risk of postoperative pulmonary complications following colorectal, gynecological, and joint arthroplasty surgeries.13-15 The Federal Drug Administration has issued a black box warning that coadministration of gabapentin or pregabalin with other sedating medications increases the risk for severe respiratory complications.16 Given that recent meta-analyses have found that gabapentin and pregabalin are only weak analgesics when used during surgery17 and with evidence showing their potential to cause serious OIRD,5,10,11,13-15 the continued use of these medicines in ERAS protocols should be questioned.
ANESTHESIA RECOVERY
In many ways a patient’s course through PACU recovery can provide the most important information regarding OIRD risk on the general care wards. Patients who have PACU respiratory depression have higher rates of postoperative pulmonary complications, and as many as one third of patients who have both a positive OSA screen and PACU respiratory depression develop postoperative pulmonary complications.9 Further, the Mayo naloxone studies found that patients who have PACU respiratory depression9 have five-fold increased risk for naloxone administration.5,6 Another study which examined the postoperative course of patients administered naloxone in the PACU who then were discharged to general care wards found that these patients had a three-fold increase risk of postoperative adverse events compared to patients who did not receive naloxone in the PACU.18
One possible explanation for the association between PACU respiratory depression and adverse respiratory events following discharge (even though PACU discharge criteria had been fulfilled) is that respiratory depression occurring during anesthesia recovery may persist on the ward. This was demonstrated in a study that used bioimpedance to continuously monitor minute ventilation of 119 patients admitted to the PACU and then for the first 12 postoperative hours on the general wards.19 Those patients who had depressed minute ventilation in the PACU continued to do so for about 10 hours on the ward. In contrast, those patients who had normal minute ventilation in the PACU mostly continued to have normal minute volume on the wards.
PRESENTATION OF OIRD
Postoperative OIRD often develops in ways which are surprising to most anesthesia professionals, both as to time of onset and presenting signs and symptoms. Understanding these concepts will help develop better postoperative monitoring plans.
A common belief is that critical OIRD events occur late at night when opioid analgesics, other sedating medications, and underlying OSA combine during sleep to create a lethal mix. A secondary analysis of PRODIGY found that the time relationship between OIRD, surgery, and time of day is more complex.20 In that study, almost all patients who had postoperative OIRD began to have multiple episodes of OIRD in the late afternoon and early evening (16:00–22:00) shortly after arriving on the wards. The frequency of OIRD episodes surged during the early morning hours (02:00–06:00).20 However, in the Mayo Clinic naloxone studies5,6 naloxone was typically administered during the afternoon and evening.4 These studies suggest it is the first few hours of admission to the ward that are most hazardous. Therefore, monitoring for OIRD should begin upon admission to the ward and not wait until bedtime.
Another common belief is that OIRD usually presents as bradypnea and/or hypoxemia. However, studies which examined nursing notes preceding severe episodes of OIRD have found that oftentimes normal respiratory rates and oxygen saturations are documented.21,22 There are several potential explanations for these findings. One is that severe OIRD develops suddenly, and, thus. signs of respiratory depression are not present during preceding vital signs checks. Research does not support this possibility. Postoperative OIRD persists for hours following PACU discharge,19 and PRODIGY showed that patients usually have multiple, repetitive OIRD events.20 A more likely possibility as to why nursing notes are often falsely reassuring is that OIRD does not present as bradypnea or oxygen desaturation. The capnography and pulse oximetry used in PRODIGY paints a different picture of OIRD than is commonly assumed.7,20 In PRODIGY, almost 100% of OIRD episodes consisted, in part, of an apnea or partial apnea event, and isolated bradypnea or oxygen desaturation were extremely rare (Figure 1).7,20 Though not shown, patients who were on supplemental oxygen and had OIRD, oftentimes did not have periods of oxygen desaturation during apnea spells. In the setting of a repetitive apnea OIRD breathing pattern, it is plausible that when a nurse comes to make an assessment, the patient will awaken to the point that normal breathing resumes, thus masking signs of respiratory depression. It is important to note that in many cases of severe OIRD, the nursing notes, while not recording signs of respiratory depression, will note that a patient is somnolent or sedated.21,22 These observations suggest that nurses should be trained to quietly observe breathing patterns of sleeping patients to assess respiratory status before measuring other vital signs that may awaken the patient such as blood pressure measurement. The fact that many patients who developed critical OIRD events were noted to be somnolent or sedated beforehand also presents an opportunity to educate nursing staff that such sedated patients should be considered higher risk and more carefully monitored.
A PROPOSED NEW APPROACH TO POSTOPERATIVE OIRD
Findings from these recent studies can allow the anesthesia professional to expand the assessment of OIRD risk beyond a preoperative OSA screen (Figure 2).8 In addition to a mandatory preoperative screening of patients for OSA,2,3 risk for OIRD should consider advancing age and overall disease burden. Calculating the PRODIGY score for OIRD risk is easy, convenient, and can be incorporated into electronic health record platforms.7
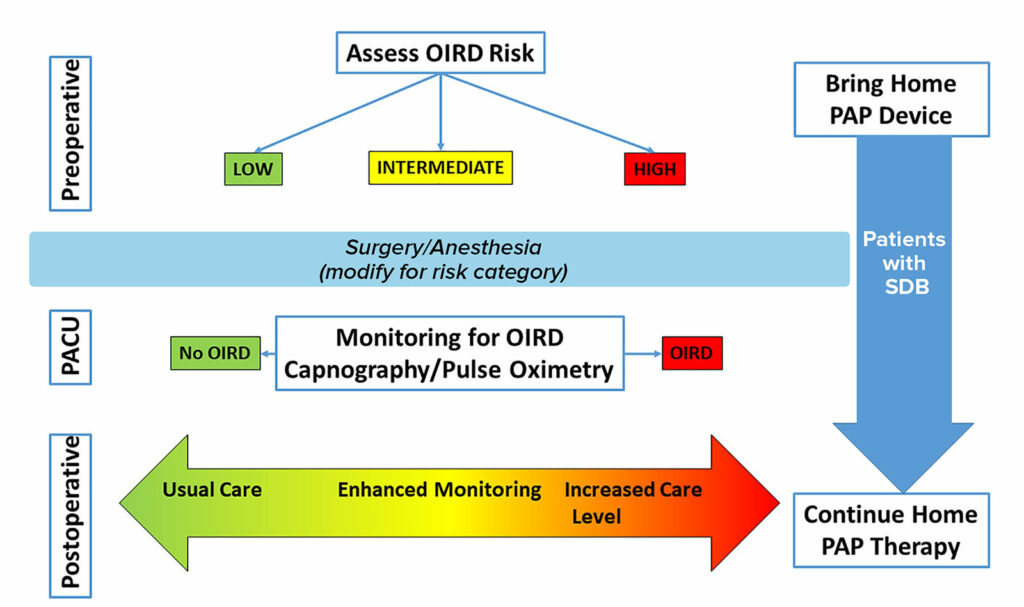
Figure 2: Proposed Clinical Pathway for Patients with Postoperative Opioid-Induced Respiratory Depression.
Clinical decisions on the postoperative level of care are complex and unique for each patient. Preoperatively, patients should have a risk assessment for respiratory depression. The surgical and anesthetic management should be tailored for this risk. During anesthesia recovery, patients’ respiratory status should be monitored for various signs of respiratory depression. Postoperative management decisions regarding level of monitoring and care should be guided by preoperative status, intraoperative status, and the anesthesia recovery course. Home therapies for sleep-disordered breathing should be continued into the postoperative period. PACU indicates postanesthesia care unit; OIRD, opioid induced respiratory depression; SDB, sleep disordered breathing; PAP, positive airway pressure.
Patients with OSA should continue to use their continuous positive airway pressure or other devices in the postoperative period.2,3 The anesthetic could be modified for higher risk patients utilizing regional blocks, shorter acting agents, and nonsedating analgesics (e.g., acetaminophen). Finally, during anesthesia recovery, patients should be monitored for episodes of respiratory depression.5,6,9 Based on this information as well as the extent of the surgical procedure, the anesthesia professional could tailor the postoperative care plan based on level of risk in regards to postoperative disposition and level of monitoring where patients deemed higher risk for OIRD are specifically targets for escalation of postoperative care.
REFERENCES
- Weinger MB, Lee LA. “No patient shall be harmed by opioid-induced respiratory depression.” APSF Newsletter. 2011;26:21–40. https://www.apsf.org/article/no-patient-shall-be-harmed-by-opioid-induced-respiratory-depression/. Accessed March 17, 2023
- Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2014;120:268–286. PMID: 24346178
- Memtsoudis SG, Cozowicz C, Nagappa M, et al. Society of Anesthesia and Sleep Medicine guideline on intraoperative management of adult patients with obstructive sleep apnea. Anesth Analg. 2018;127:967–987. PMID: 29944522
- Weingarten TN, Sprung J. An update on postoperative respiratory depression. Int Anesthesiol Clin. 2022;60:8–19. PMID: 35261341
- Deljou A, Hedrick SJ, Portner ER, et al. Pattern of perioperative gabapentinoid use and risk for postoperative naloxone administration. Br J Anaesth. 2018;120:798–806. PMID: 29576120
- Weingarten TN, Herasevich V, McGlinch MC, et al. Predictors of delayed postoperative respiratory depression assessed from naloxone administration. Anesth Analg. 2015;121:422–429. PMID: 25993390
- Khanna AK, Bergese SD, Jungquist CR, et al. Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective, observational trial. Anesth Analg. 2020;131:1012–1024. PMID: 32925318
- Weingarten TN, Sprung J. Review of postoperative respiratory depression: from recovery room to general care unit. Anesthesiology. 2022;137:735–741. PMID: 36413782
- Gali B, Whalen FX, Schroeder DR, et al. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology. 2009;110:869–877. PMID: 19293694
- Cavalcante AN, Sprung J, Schroeder DR, Weingarten TN. Multimodal analgesic therapy with gabapentin and its association with postoperative respiratory depression. Anesth Analg. 2017;125:141–146. PMID: 27984223
- Weingarten TN, Jacob AK, Njathi CW, et al. Multimodal analgesic protocol and postanesthesia respiratory depression during phase I recovery after total joint arthroplasty. Research support, non-U.S. Gov’t. Reg Anesth Pain Med. 2015;40:330–336. PMID: 25967650
- Weingarten TN, Bergan TS, Narr BJ, et al. Effects of changes in intraoperative management on recovery from anesthesia: a review of practice improvement initiative. BMC Anesthesiol. 2015;15:54. PMID: 25902828
- Ohnuma T, Krishnamoorthy V, Ellis AR, et al. Association ‘between gabapentinoids on the day of colorectal surgery and adverse postoperative respiratory outcomes. Ann Surg. 2019;270:e65–e67. PMID: 30985370
- Ohnuma T, Raghunathan K, Moore S, et al. Dose-dependent association of gabapentinoids with pulmonary complications after total hip and knee arthroplasties. J Bone Joint Surg Am. 2020;102:221–229. PMID: 31804238
- Tan HS, Frere Z, Krishnamoorthy V, et al. Association of gabapentinoid utilization with postoperative pulmonary complications in gynecologic surgery: a retrospective cohort study. Curr Med Res Opin. 2021;37:821–828. PMID: 33685298
- FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR): When used with CNS depressants or in patients with lung problems. FDA Drug Safety Communication. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin. Accessed December 19, 2019.
- Verret M, Lauzier F, Zarychanski R, et al. Perioperative use of gabapentinoids for the management of postoperative acute pain: a systematic review and meta-analysis. Anesthesiology. 2020;133:265–279. PMID: 32667154
- Weingarten TN, Chong EY, Schroeder DR, Sprung J. Predictors and outcomes following naloxone administration during Phase I anesthesia recovery. J Anesth. 2016;30:116–122. PMID: 26449674
- Schumann R, Harvey B, Zahedi F, Bonney I. Minute ventilation assessment in the PACU is useful to predict postoperative respiratory depression following discharge to the floor: A prospective cohort study. J Clin Anesth. 2019;52:93–98. PMID: 30227321
- Driver CN, Laporta ML, Bergese SD, et al. Frequency and temporal distribution of postoperative respiratory depressive events. Anesth Analg. 2021;132:1206–1214. PMID: 33857962
- Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122:659–665. PMID: 25536092
- Valencia Morales DJ, Laporta ML, Meehan AM, et al. Incidence and outcomes of life-threatening events during hospitalization: a retrospective study of patients treated with naloxone. Pain Med. 2022;23:878–886. PMID: 34668555


Leave a Reply
You must be logged in to post a comment.