Methadone is a mu agonist used in detoxification treatment for patients who abuse opioids and to treat those in chronic pain.1 Methadone is also an N-methyl-D-aspartate (NMDA) receptor antagonist, which makes it a unique drug for management of neuropathic pain and opioid-induced hyperalgesia.2 It has been shown that patients on methadone treatment can experience prolongation of the corrected QT (QTc) interval on the electrocardiogram (ECG), which in a subset of patients can lead to malignant arrhythmias such as torsades de pointes (TdP) and sudden cardiac death.3
Case
We present the case of a 72-year-old woman with a history of hypertension, diabetes mellitus type 2, stroke, bilateral chronic knee pain and opioid addiction. She was admitted to the emergency department after a syncopal episode. She was unresponsive and required endotracheal intubation on arrival.
The patient was transferred to the ICU to manage her electrolyte imbalance (i.e., hypokalemia and hypomagnesemia) and hemodynamic instability in the setting of acute kidney failure. Monitoring by ECG revealed a prolonged QTc interval (552 msec), followed by polymorphic ventricular tachycardia (i.e., TdP) that progressed to cardiac arrest, requiring resuscitation on two occasions. At that time, the cardiac dysrhythmias were assumed to be secondary to electrolyte derangements. After extubation, further interrogation of the patient obtained a history of chronic methadone use for opioid addiction as well as knee pain.
The patient was discharged home after 12 days on a lower dose of methadone. However, she was readmitted a week later with another, similar syncopal episode. A subsequent ECG showed atrial flutter with rapid ventricular response, premature ventricular contractions (PVCs) and a prolonged QTc interval (574 msec), but with no electrolyte abnormalities. She had one more episode of syncope or seizure-like activity, and required placement of a temporary transvenous pacemaker during this hospitalization.
Our pain team was consulted to manage the patient’s knee pain. Methadone was assumed to be the causal agent for her arrhythmias and was discontinued. The patient was started on codeine and gabapentin for pain control. The addiction team was consulted for treatment using buprenorphine. A follow-up ECG four months later showed a normal sinus rhythm with a QTc interval of 477 msec.
Methadone as Pain Medicine: A Review
Materials and Methods
The content of this review was extensively collected from Google Scholar database, PubMed and ScienceDirect, for which search terms included methadone, pharmacology, NMDA, methadone side effects, QTc, torsades de pointes and morphine methadone nonlinear dose conversions, and the years covered were 1995 to 2019.
Clinical Use
Methadone is a synthetic, long-acting opioid pharmacologically similar to morphine. It is used extensively to treat pain and as opioid maintenance therapy for detoxification in people with opioid dependence.1 The drug was not used clinically for several years because the high dosages used in preliminary testing caused substantial side effects. During the 1950s, however, methadone emerged as a treatment for opioid addiction, and it has remained the primary therapy for this condition.
Methadone is classified as a Schedule II drug. With its multiple receptor site functions, it is used to palliate cancer pain and relieve chronic pain states. In addition, acute pain patients who are resistant to routine pain management options may respond well to methadone because of its unique pharmacokinetic and pharmacodynamic activity. Moreover, methadone has convenient and numerous routes of administration, is relatively inexpensive and has a long half-life, properties that make it a useful and viable option for many patient populations.
Prescribing methadone for opioid dependence or withdrawal in the United States has been legal in the setting of federally licensed methadone maintenance facilities since the enactment of the Narcotic Addict Treatment Act of 1973-1974.2 Today, more than 150,000 opioid-dependent individuals are enrolled in methadone treatment centers in the United States.
History
Methadone was developed in the early 1930s in the laboratories of Hoechst AG by two German scientists, Gustav Ehrhart and Max Bockmühl. This synthetic substance was originally called “Polamidon” or “Hoechst 10820.” Though synthesized in the 1930s, it was first approved by the United States’ FDA in 1947 as an analgesic when it was introduced by Eli Lilly and Company under the trade name “Dolophine.” However, later in 1947, researchers realized the addictive potential of this drug and its use as a pain medication fell out of favor. Eventually, in 1965, methadone was approved for maintenance treatment in opioid-addicted patients, and later was added to the World Health Organization’s Model List of Essential Medicines.3
Pharmacology and Mechanism of Action
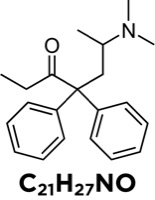
Methadone is a synthetic opioid in the structural class of diphenylpropylamines (Figure 1).
Methadone hydrochloride is a mu-receptor agonist and an NMDA-receptor antagonist. Its actions mimic the endogenous opioids, encephalin and endorphins. The D isomer is an NMDA antagonist and the L isomer is a mu-opioid receptor agonist.4 The analgesic property of methadone is a result of its high affinity for mu receptors (key mediators in supraspinal analgesia) and delta receptors (important in spinal analgesia). NMDA receptors open in response to the excitatory neurotransmitter glutamate, which is released with exposure to noxious stimuli. The activation of NMDA receptors has been associated with hyperalgesia, neuropathic pain and reduced functionality of opioid receptors, leading to central sensitization, wind-up and tolerance. Methadone abolishes hyperalgesia and opioid tolerance due to its NMDA antagonist action.
Methadone’s biochemical properties make it a good option both to control neuropathic pain and to use in opioid addiction treatment. The enantiomer S-methadone prevents the reuptake of serotonin and norepinephrine. With its combination of opioid agonism and NMDA receptor antagonism, methadone provides significant analgesia with fewer side effects compared with other commonly used opioids.
Methadone binds to opioid receptors in the central nervous system, causing inhibition of ascending pain pathways and altering the perception of and response to pain. It also affects the release of other neurotransmitters, such as acetylcholine, norepinephrine, substance P and dopamine.5 These actions account for analgesia, antitussive properties, hypotension, respiratory depression, sedation, decreased bowel motility, increased prolactin, increased release of growth hormone and miosis. Prolonged use of methadone results in partial tolerance to most pharmacologic effects. A milder form of abstinence syndrome, consisting of rhinorrhea, sneezing, nausea, vomiting, lacrimation, fever, chills, tremor and tachycardia, occurs on abrupt discontinuation of the drug or the administration of an antagonist such as naloxone hydrochloride. Because of its long half-life and duration of action, the abstinence syndrome is delayed and prolonged, but less severe than that from shorter-acting opiates.
Pharmacokinetics
Routes of administration: Methadone is marketed as a lipophilic hydrochloride salt. It is available for oral, rectal, subcutaneous, epidural, intrathecal and parenteral administration. It is well absorbed by all routes.6,7
Absorption: Methadone has more than 80% oral and rectal bioavailability, compared with 26% for morphine.8 Methadone is absorbed rapidly from the stomach, with little absorption occurring past the pylorus. Oral administration is followed by rapid gastrointestinal absorption, with measurable levels in the plasma at 30 minutes. Decreased gastric emptying in chronic users can slow time to peak levels. Following absorption, methadone is distributed to the brain, liver, kidneys, lungs and muscles.5 Following oral administration, the bioavailability of methadone ranges from 36% to 100%, and peak plasma concentrations are reached at between 1 and 7.5 hours. These ranges are due to individual patient variation in cytochrome enzyme CYP3A4 expression or polymorphisms in CYP2D6 among different patient populations.9
Distribution: Methadone is a lipophilic drug and is widely distributed in the tissues. The drug is primarily bound to alpha-1-acid glycoprotein (85%-90%).10 Extensive protein and tissue binding is responsible for the prolonged half-life of the drug following continuous usage. Blood concentrations of alpha-1-acid glycoprotein increase in conditions of stress and in heroin addicts, which can result in increased levels of bound drug that may fall slowly over time.9 Tissue binding results in accumulation of the drug with repeated dosing, and its reabsorption from the tissues continues for weeks after administration has ceased. No relationship has been established between the plasma concentration and analgesic effect, which is why methadone for pain management should be titrated to effect rather than drug level. Methadone is secreted in saliva, breast milk, amniotic fluid and umbilical cord plasma.
Metabolism: Elimination of methadone occurs by hepatic oxidative biotransformation, renal N-demethylation, and urinary and fecal clearance. Long-term administration results in increased metabolite-to-methadone ratios, suggesting that auto-induction of hepatic microsomal enzymes occurs. Methadone is primarily metabolized in the liver, with no active metabolites, by N-demethylation to the metabolite 2-ethylidene-1,5-dimethyl-3, 3-diphenylpyrrolidene (EDDP). Cytochrome P450 (CYP450) enzymes, primarily CYP3A4, CYP2B6 and CYP2C19, and to a lesser extent CYP2C9 and CYP2D6, are responsible for the conversion of methadone to EDDP and other inactive metabolites, which are excreted mainly in the urine. Coadministration with CYP inducers may result in more rapid metabolism and the potential for decreased drug effects, whereas administration with CYP inhibitors may reduce metabolism and potentiate methadone’s effects.
Patients with chronic, stable liver disease can tolerate the usual dose of methadone for maintenance therapy. Renal impairment does not impair clearance, so methadone may be useful for pain management in patients with renal failure.
Elimination and analgesic half-life: Methadone has a prolonged half-life due to high protein binding. Its half-life is approximately 22 to 100 hours and analgesic half-life is four to eight hours.14 Approximately 4.5 days is required for methadone to reach a steady state, meaning the same amount of medication ingested is the same amount of medication being eliminated. In some patients, the elimination half-life may be as long as 60 hours, which would take 12.5 days for methadone to reach the steady state. Rapid dose escalation may cause sedation and respiratory depression, so an individual should wait about five to seven days before increasing the dose.
Excretion: Elimination of methadone is mediated by extensive biotransformation, followed by fecal and renal excretion. It usually undergoes a biphasic pattern of elimination: slow distribution or alpha elimination (eight to 12 hours) followed by a longer beta phase (30-130 hours). The alpha elimination (binding to lipophilic tissue) correlates with the duration of analgesia, which is typically six to eight hours. The plasma level in the beta elimination phase is subanalgesic but sufficient to prevent withdrawal symptoms. Unlike morphine, it usually is not necessary to adjust the methadone dose in patients with renal insufficiency. Methadone may be used safely in renal disease because 45% of the drug and its metabolites are eliminated through the fecal route. Some experts still suggest a 50% reduction in methadone dosing since 11% of methadone is typically excreted via the renal route, and this proportion may go up to 57% when urinary pH is less than 6. Methadone is highly bound to protein, so dialysis does not increase the elimination of the drug.15
Drug-drug interactions are listed in Table 1.
| Table 1. Drug Interactions With Methadone | |||||
| Serum Levels Increased by Methadone |
|
||||
|---|---|---|---|---|---|
| Reduced Methadone Clearance |
|
||||
| Increased Methadone Clearance |
|
||||
| CNS Depression |
|
||||
| QTc Prolongation |
|
||||
| Serotonin Syndrome |
|
||||
| 5-HT3, serotonin; CNS, central nervous system; MAO, monoamine oxidase; SNRIs, serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants; QTc, corrected QT
Based on references 11-13.
|
|||||
Therapeutic Uses
The principal therapeutic uses of methadone are for analgesia and opioid maintenance therapy.
1. Role of Methadone in Pain Management
In addition to being a mu-opioid receptor agonist and NMDA antagonist, methadone inhibits the reuptake of serotonin and norepinephrine. Methadone is an effective pain medication for chronic cancer pain (mu- opioid receptor activation)16 and chronic neuropathic pain (NMDA receptor antagonist effects).17-20 For patients on high doses of opioid analgesics, methadone is a good alternative for pain control due to its reduced opioid tolerance development. In a study of cancer patients who had pain refractory to opioids or experienced side effects, 80% reported good pain relief and fewer side effects following a switch to methadone.21
As a powerful NMDA receptor blocker, methadone blocks synaptic plasticity in patients with chronic pain. The federal and state regulations that restrict the use of methadone in addiction management do not apply when methadone is used for chronic pain. Although methadone has a long half-life, no relationship exists between plasma concentration and its analgesic effects. Frequent daily dosing is usually required for pain relief. Caution should be taken not to increase the dose too high or too frequently when initiating or optimizing therapy because toxic effects, such as excessive sedation and respiratory depression, may ensue. Benzodiazepines, barbiturates and alcohol have an additive effect on respiratory depression and should be avoided in patients taking methadone. Since it has no cross-reactivity, methadone can also be used in patients with morphine allergy.21
NMDA Receptor and NMDA Antagonists
The NMDA receptor is a glutamate and ion channel receptor found widely in the nervous system. It is activated by glutamate, which is a major excitatory synaptic neurotransmitter. Another kind of glutamate receptor is the AMPA receptor. AMPA and NMDA receptors often work in conjunction to produce long-lasting changes in synaptic functioning, plasticity and memory (Figure 2).

The opening and closing of the NMDA receptor is complex. While it is primarily a ligand-gated channel, it also has weaker voltage-dependent modulation. Glutamate is released by the presynaptic neuron in response to noxious stimuli and is excitatory at AMPA and NMDA receptors. AMPA receptor activation by glutamate allows sodium ions to flow into the neuron, depolarizing the membrane. This depolarization removes the magnesium ion block, resulting in an influx of calcium ions through the NMDA receptor channel. The NMDA receptor acts differently from most receptor molecules because it is both ligand-gated and voltage-sensitive.
Structural and biochemical changes occur at the neuronal synapse in pain conditions because of repeated nociceptor stimulation and transmission of impulses. These changes result in synaptic plasticity, which includes an alteration in the number of receptors located on the synapse leading to wind-up. The NMDA receptor is involved in the development of chronic pain due to synaptic plasticity and because of its capacity to allow entry of intracellular calcium on activation.
Activation of the NMDA receptor has been associated with hyperalgesia, neuropathic pain and reduced function of opioid receptors. Hyperalgesia and neuropathic pain result from increased neuronal sensitization, leading to a heightened level of pain. The reduced function of opioid receptors is caused by a decrease in the opioid receptor’s sensitivity that translates to opioid tolerance, as patients will require higher doses of opioids to achieve the same therapeutic effects. NMDA antagonists may have a role in these areas of pain management. Examples of NMDA receptor antagonists are ketamine, methadone, memantine, amantadine and dextromethorphan.
Methadone has been extensively studied in opioid tolerance and neuropathic pain conditions.22 It has been shown to be a good replacement opioid in patients who are poorly controlled or experience dose-limiting adverse effects while on other opioids. In 80% of cancer patients with uncontrolled pain or significant side effects with morphine, a rotation to methadone demonstrated reductions in pain and adverse effects.21 Methadone also showed efficacy in patients with refractory neuropathic pain.
Methadone Dosing for Pain Management
Methadone is available in 2.5-, 5- and 10-mg tablets. Methadone dosing depends on whether the patient is opioid-naive or tolerant. IV dosing is twice as effective as the oral route. For acute severe pain, methadone is dosed at 2.5 to 10 mg every three to four hours. In older and debilitated patients, the recommended dose is 2.5 mg per day orally. For severe chronic cancer pain and neuropathic pain, 5 to 20 mg every six to eight hours is recommended.
Guidelines for prescribing methadone to opioid-tolerant patients:
- Determine the total daily dose of the opioids.
- Convert that number to an equivalent dose of morphine (morphine milligram equivalent or MME).
- Due to methadone’s property of abolishing opioid tolerance, the conversion of equivalent analgesic doses of morphine to methadone does not follow a linear correlation and caution should be exercised when switching from morphine to methadone (Table 2).
- Start at 25% of the total daily morphine dose. Some experts recommend stopping morphine completely and starting low-dose methadone every six to eight hours, and adding a short-acting opiate for breakthrough pain.23
| Table 2. Conversion Ratio From Morphine to Methadone24 | |
| Daily Morphine Dose (MME) | Morphine to Methadone Conversion Ratio |
|---|---|
| <100 mg | 4:1 (25%) |
| 100-300 mg | 8:1 (12.5%) |
| 300-600 mg | 10:1 (10%) |
| 600-800 mg | 12:1 (8.3%) |
| 800-1,000 mg | 15:1 (6.7%) |
| >1,000 mg | 20:1 (5%) |
| MME, morphine milligram equivalents
Based on CDC guidelines.
|
|
2. Role of Methadone in Opioid Addiction Maintenance Treatment
Maintenance therapy is the long-term administration of methadone hydrochloride to patients who are dependent on opiates. Dole and Nyswander first used this synthetic opioid in the United States as a treatment for narcotic addiction in 1965.25 There is cross-tolerance and cross-dependence among the various opiates. This is the foundation for using methadone in the detoxification and maintenance of people addicted to heroin.
Methadone withdrawal syndrome, although similar to that of morphine, differs in that the onset is slower, the course is more prolonged, and the symptoms are less severe. The aim is to substitute methadone, a legal, oral opiate with a long half-life, for the illicit, parenterally administered heroin, which is associated with a high risk for morbidity and mortality.
Methadone prevents opioid withdrawal symptoms, attenuates the euphoric effects of heroin, and minimizes the craving for heroin. Methadone maintenance has been shown to be a safe and cost-effective treatment that reduces illicit heroin use, decreases the incidence of bloodborne infections (hepatitis B, HIV, etc.), reduces criminal activity, improves social outcomes, and reduces overall morbidity and mortality.26-31 The composition of street heroin may be variable, so empiric methadone dosing is recommended over equivalent dosing based on recent heroin use.30
The dose of 5 mg of parenteral heroin is approximately equivalent to 20 mg of oral methadone, but the dosage of methadone for maintenance therapy is variable. It is usually started at 10 to 20 mg daily and increased slowly in 10-mg increments until withdrawal symptoms are well controlled. Most patients can be maintained at 30 to 40 mg per day to control withdrawal symptoms.30 Treatment effectiveness varies among individuals and long-term therapy may be required. Methadone maintenance treatment enables a person to function as a productive member of society by decreasing craving and the constant preoccupation with obtaining heroin.
3. Role of Methadone in Attenuating Tolerance and Opioid-Induced Hyperalgesia
Tolerance is the pharmacologic concept of an inverse relationship of response to a drug and dosing. It can occur with a variety of drugs and is not limited to opioids. In simple terms, it is the shift of the dose-response curve to the right. Activation of NMDA receptors is involved in the development of tolerance, hypersensitivity and central sensitization. By inhibiting NMDA receptors, methadone leads to desensitization and prevention of opioid tolerance.
Opioid-induced hyperalgesia (OIH) is a form of pain sensitization caused by an opioid drug, in which increasing the dose worsens pain and reducing the dose improves pain.32 OIH has been observed in patients receiving morphine for pain, and was described by Albut and his colleagues in 1870.33 In 1880, Rossbach noted that “when dependence on opioids finally becomes an illness of itself, opposite effects like restlessness, sleep disturbance, hyperesthesia, neuralgia, and irritability become manifest.”34 OIH is defined as a state of nociceptive sensitization caused by opioid exposure, and seems to be related to opioid tolerance. The pain experienced may be an exacerbation of underlying pain or might be a different pain leading to loss of opioid efficacy. It is generally thought to exist due to neuroplastic changes in both the peripheral and central nervous system that lead to sensitization of pro-nociceptive pathways. Various mechanisms described in the development of OIH include10:
- NMDA receptors, when blocked, prevent the development of tolerance and OIH.
- The glutamate transporter system is inhibited in OIH, thereby increasing the amount of glutamate available to the NMDA receptors.
- Calcium-regulated intracellular protein kinase C is likely a link between cellular mechanisms of tolerance and OIH.
- There may be crosstalk between the neural mechanisms of pain and tolerance.
- Prolonged morphine administration induces neurotoxicity via NMDA receptor–mediated apoptotic cell death in the dorsal horn.
OIH should be considered when the opioid’s effectiveness seems to be waning in the absence of disease progression. If there is unexplained pain, diffuse allodynia or increased levels of pain with increasing dosages, OIH should be considered. Treatment involves reducing the opioid dosage, tapering, or supplementing with NMDA receptor modulators.
Methadone Use in Special Populations
Pregnant Women
Many pregnant women in the United States test positive for opioid use at the time of admission to the hospital for delivery.35 Methadone is considered the standard of care for management of opioid addiction in these patients. Pregnant women on methadone receive better prenatal care than untreated women. This results in higher infant birth weights and fewer obstetric complications and preterm births, as well as less neonatal morbidity.
Women receiving methadone for opioid addiction should be maintained on their daily dose of methadone in addition to receiving the same pain management options during labor and delivery as opioid-naive patients. Methadone crosses the placenta and can be detected in cord blood, amniotic fluid and newborn urine. The concentration in the umbilical cord is one-fourth that in maternal serum. Chronic maternal methadone treatment may reduce the fetal heart rate variability during the first stage of delivery.35
The neonate of a chronic opioid user should be monitored for neonatal withdrawal syndrome, which can be life-threatening if not recognized and treated. Close monitoring of the neonate is crucial because withdrawal may be observed even up to two to four weeks after delivery. Neonatal abstinence syndrome following opioid exposure may present with autonomic (e.g., fever, temperature instability), gastrointestinal (e.g., diarrhea, vomiting, poor feeding/weight gain), or neurologic (e.g., high-pitched crying, increased muscle tone, irritability, seizure, tremor) symptoms.
Breastfeeding Women
Infants of nursing mothers on methadone can present with mild sedation to respiratory depression, as 2% to 3% of the maternal dose of the drug is secreted in breast milk. Peak methadone levels appear in breast milk four to five hours after an oral dose. It is recommended that nursing mothers observe their infants for sedation and contact their health care provider for emergency care if the need arises.36 It is also recommended to slowly wean infants off breastfeeding to prevent withdrawal symptoms in them. If additional illicit substances are being used, women treated with methadone should pump and discard breast milk until sobriety is established.
Methadone Treatment and Infants
The newborns of mothers on long-term methadone therapy are at higher risk for sudden infant death syndrome. Although there is a transient, benign increase in the QT interval in healthy newborns, maternal methadone use prolongs QTc in infants in the first two days of life, similar to the effects of methadone in adults. Methadone is highly lipophilic and has high protein binding, so its distribution and metabolism are affected in neonates since they have a higher fat content and higher plasma protein concentrations.37 Elimination of methadone also is slower in neonates.
Adverse Effects of Methadone
The action of methadone at opioid receptors can lead to opioid-related side effects, including sedation, respiratory depression and constipation. Methadone can cause serotonin syndrome when given with other serotonergic medications, such as monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and certain tricyclic antidepressants. Life-threatening side effects include respiratory depression and cardiac effects such as QT prolongation,38 QT dispersion, pathologic U wave and Brugada-like syndrome.
Boxed Warning for Methadone: QT Prolongation and TdP
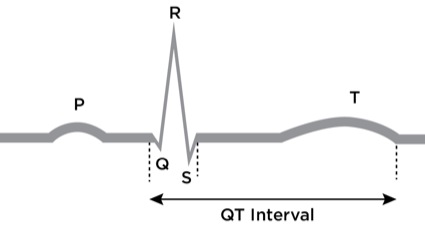
QT prolongation is common in patients receiving methadone maintenance.39,40 The QT interval, which is the time between the start of the Q wave and the end of the T wave (Figure 3), generally is corrected (QTc) for its natural dependence on heart rate using Bazett’s formula: QTc = QT interval divided by the square root of the preceding RR interval. Although this formula is likely to over-correct in the setting of a high heart rate, it is still a reasonable method for screening purposes. A QT interval of 390 to 420 milliseconds (msec) in men or 400 to 440 msec in women is generally considered normal. Prolonged QT interval has been defined as greater than 450 msec in men and greater than 470 msec in women. Prolongation of the QT interval occurs when there is an interruption in the normal balance and flow of ions in the myocardium, increasing the time required for repolarization to occur.
The cause of prolonged QT interval and TdP is the blockade of the human ether-a-go-go–related gene (hERG). This gene encodes for a subunit of the voltage-gated potassium channels found predominantly in the myocardium. It was first identified in 1994 and is located on chromosome 7. These channels facilitate delayed potassium ion currents causing repolarization, an important component of phase 3 of cardiac action potential. Blockade of these ion channels prolongs the terminal portion of the cardiac action potential, causing delayed repolarization, which manifests as QT interval prolongation as seen on the ECG.
Methadone is a potent inhibitor of the hERG channel in clinical doses. Methadone’s r isomer inhibits the hERG channel less than the S isomer.41 S-methadone binds to the hERG to cause QT prolongation, which can lead to TdP and sudden cardiac death. Coadministration of triggering drugs that can prolong the QT interval or result in increased levels of S-methadone increases the risk for QT prolongation. The risk for TdP begins at a QTc interval of 450 msec and is significant at more than 500 msec, or an increase in QTc of more than 60 msec from baseline.
Methadone exerts negative chronotropic effects by two important mechanisms: calcium channel antagonism and anticholinesterase properties. Bradycardia (<40 beats per minute) caused by methadone increases the risk for TdP, an effect that has been confirmed clinically. The risk factors for QT prolongation and TdP include low serum potassium, magnesium or calcium concentrations; diabetes mellitus; thyroid or pituitary insufficiency; cardiomyopathy; recent myocardial infarction; sinus bradycardia; certain drugs that can prolong QT; toxins (organophosphates, insecticides and heavy metals); subarachnoid hemorrhage; starvation; older age; female sex; genetic susceptibility; obesity; alcoholism; and cirrhosis. Concomitant use of cocaine and methadone can prolong the QT interval via the same mechanisms.42
QT Interval Dispersion
The spatial dispersion of ventricular recovery times is indicated by QT dispersion, which equals a maximum QT interval minus a minimum QT interval. It is an approximate measure of a general abnormality of repolarization.43 Methadone can increase QT dispersion in addition to QT interval prolongation. The normal range for QT dispersion is recorded as 30 to 60 msec.
Pathologic U Wave
Methadone can cause pathologic U waves on a 12-lead ECG. The occurrence of an abnormal U wave might indicate impending TdP or ventricular fibrillation. Drug-induced or drug-prolonged QT syndromes can generate U waves. In a study on the effects of methadone and buprenorphine on the ECG, patients taking methadone were significantly more likely to have U waves and ventricular bigeminy.44
Brugada-Like Syndrome
Brugada-like syndrome is a rare condition in patients on methadone, predisposing them to life-threatening ventricular tachycardia and sudden cardiac death.45 This adverse event is explained as a mutation in the cardiac sodium channel SCN5A gene in 25% of cases. Causes of Brugada-like rhythms include cocaine, methadone, sodium channel blockers (e.g., bupivacaine, flecainide, lidocaine, procainamide, propafenone and tricyclic antidepressants), propofol and electrolyte imbalances. An abnormal 12-lead ECG with coved-type ST elevation in precordial leads V1 to V3 is characteristic of the syndrome.
Guidelines for Safe Methadone Prescribing
Providers caring for patients on methadone should be familiar with the risk factors associated with methadone-induced prolongation of the QTc interval and comply with the safety guidelines for prescribing methadone.46
- Patient assessment: All patients should be evaluated for risk factors for QT prolongation prior to initiating methadone. The new patient evaluation should include a complete medication history; personal and family history of structural heart disease (including long QT syndrome, sudden cardiac death, myocardial infarction, heart failure); personal history of arrhythmia and/or syncope; and use of QT-prolonging drugs, including prescribed medications and illicit drugs such as cocaine.
- Modifiable risk factors should be corrected. Serum electrolytes should be measured regularly, particularly in patients with vomiting or diarrhea or those taking diuretics, and in renal failure patients.
- Education and counseling: Patients and family members should be counseled about potential risks and benefits before initiating methadone therapy. Patients should take methadone as prescribed and comply with recommended follow-up and monitoring. Patients should be informed of the symptoms of arrhythmia and told to seek medical advice promptly if they occur.
- Low beginning dose: Start methadone at low doses (≤40 mg daily) and titrate up slowly to reduce the risk for accidental overdose. Careful dose titration is required due to methadone’s long half-life and pharmacokinetic variability. Maintain patients on the lowest effective dose of methadone.
- Alternative medications: In patients with QTc interval prolongation while receiving methadone therapy, the dose should be reduced and any concomitant risk factors should be corrected. Buprenorphine is the alternative therapy approved by the FDA since it has less effect on cardiac potassium channels. Morphine and codeine appear to have little effect on cardiac potassium channels, so these agents may be more appropriate choices for opioid pain management.
- Baseline ECG: All patients should have a pretreatment ECG to measure QTc interval and a follow-up ECG within 30 days, and annually thereafter. If the methadone dose is more than 100 mg per day, or if patients are older than 68 years or are female, have unexplained syncope or seizures, electrolyte abnormalities, impaired liver function, structural heart disease, genetic predisposition and previous QTc longer than 450 msec, or are taking concomitant QT-prolonging drugs, additional ECGs are recommended. If QT prolongation occurs (i.e., QTc >500 msec or an increase of >40 msec), consider reducing or discontinuing the dose of methadone, eliminating other contributing factors, and transitioning the patient to an alternative treatment such as buprenorphine.
- Urine drug testing: Perform urine drug testing before starting methadone therapy and at regular intervals for patients being treated for opioid addiction.
Other Treatment Modalities
- Buprenorphine is a partial mu-opioid receptor agonist and kappa-antagonist that is effective for decreasing opioid use among addicted people. QT prolongation has not been reported with buprenorphine, so it is a good alternative to methadone in opioid dependency.47 During the first years of methadone and buprenorphine administration, the death rate among users was compared, showing that buprenorphine was associated with a much lower mortality rate.
- In cases of recurrent methadone-associated TdP where buprenorphine cannot be used, permanent pacing with an implantable cardiac defibrillator can be done.48,49
- Another alternative treatment currently being studied and performed in patients with limitations in switching off the drug, or in those at risk for device infection, is left cardiac sympathetic denervation.50,51 This method involves a preganglionic denervation causing antiarrhythmic effects, and is being studied as an important adjunctive therapy.
Conclusion
Methadone, a relatively older drug that is coming back into the limelight due to its unique properties, is not only beneficial in management of withdrawals in opioid-dependent patients, but is also used clinically in pain management. When compared to other opioids, methadone is not only a mu-receptor agonist but has antagonist actions on the NMDA receptor, which is now realized plays an important role in maintenance of chronic pain states, OIH, and neuropathic pain. On one hand, its unique pharmacokinetic properties make methadone a useful drug for pain management and opioid addiction, but on the other hand, its long half-life and drug-drug interactions can lead to a variety of side effects. Therefore, understanding its pharmacology is essential for physicians to manage these patients in a safe and effective manner.
References
- Goldstein A, Herrera J. Heroin addicts and methadone treatment in Albuquerque: a 22-year follow-up. Drug Alcohol Depend. 1995;40(2):139-150.
- Methadone listing as new drug with special requirement and opportunity for hearing. Fed Regist. 1972;37(242):26790-26807.
- Defalque RJ, Wright AJ III. The early history of methadone: myths and facts. Bull Anesth Hist. 2007;25(3):13-16.
- Holm KM, Linnet K. Determination of the unbound fraction of R– and S-methadone in human brain. Int J Legal Med. 2016;130(6):1519-1526.
- Reisine T, Pasternak G. Opioid analgesics and antagonists. In: Hardman JG, Limbird LE, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill Book Company; 1996:544-545.
- Garrido MJ, Troconiz IF. Methadone: a review of its pharmacokinetic/pharmacodynamic properties. J Pharmacol Toxicol Methods. 1999;42(2):61-66.
- Davis MP et al., Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9(2):73-83.
- Gourlay GK et al.; A comparative study of the efficacy and pharmacokinetics of oral methadone and morphine in the treatment of severe pain in patients with cancer. Pain. 1986:25(3):297-312.
- Ferrari A, Coccia CP, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50(6):551-559.
- Lee M, Silverman S, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.
- de Castro J, Aguirre C, Rodriguez-Sasiain JM, et al. The effect of changes in gastric pH induced by omeprazole on the absorption and respiratory depression of methadone. Biopharm Drug Dispos. 1996;17(7):551-563.
- Bouer R, Barthe L, Philibert C, et al. The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: in vitro studies using the rat everted intestinal sac. Fundam Clin Pharmacol. 1999;13(4):494-500.
- Tatro DS, ed. Drug Interaction Facts: The Authority on Drug Interactions. St. Louis, MO: Lippincott Williams & Wilkins: 2005;490-493.
- Mercadante S, Sapio M, Serretta R, et al. Patient-controlled analgesia with oral methadone in cancer pain: preliminary report. Ann Oncol. 1996:7(6):613-617.
- Sunilkumar MM, Lockman K. Practical pharmacology of methadone: A long-acting opioid. Indian J Palliat Care.2018;24(5):10-14.
- National Collaborating Centre for Cancer (UK). Opioids in palliative care: safe and effective prescribing of strong opioids for pain in palliative care of adults. May 2012. https://www.ncbi.nlm.nih.gov/?pubmed/?23285502. Accessed May 29, 2019.
- Callahan R et al.: Functional inhibition by methadone of N-methyl-D-aspartate receptors expressed in Xenopus oocytes: stereospecific and subunit effects. Anesth Analg. 2004;98(3):653-659.
- De Conno F, Groff L, Brunelli C, et al. Clinical experience with oral methadone administration in the treatment of pain in 196 advanced cancer patients. J Clin Oncol. 1996;14(10):2836-2842.
- Gardner-Nix JS. Oral methadone for managing chronic nonmalignant pain. J Pain Symptom Manage. 1996;11(5):321-328.
- Nicholson AB. Methadone for cancer pain. Cochrane Database Syst Rev. 2004(2):CD003971.
- Mercadante S, Casuccio A, Fulfaro F, et al. Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol. 2001;19(11):2898-2904.
- College of Physicians and Surgeons of Ontario. Evidence-based recommendations for medical management of chronic non-malignant pain: reference guide for physicians. November 2000. http://www.globalrph.com/?narcoticonv.htm.
- Bruera E, Palmer JL, Bosnjak S, et al. Methadone versus morphine as a first-line strong opioid for cancer pain: a randomized, double-blind study. J Clin Oncol 2004;22(1):185-192.
- Ayonrinde OT, Bridge DT. The rediscovery of methadone for cancer pain management. Med J Aust. 2000;173(10):536-440.
- Dole VP, Nyswander ME. Heroin addiction—a metabolic disease. Arch Intern Med. 1967;120(1):19-24.
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93(4):515-532.
- Metzger DS, Woody GE, McLellan AT, et al. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6(9):1049-1056.
- Bell J, Mattick R, Hay A, et al. Methadone maintenance and drug-related crime. J Subst Abuse. 1997;9:15-25.
- Catalano RF, Gainey RR, Fleming CB, et al. An experimental intervention with families of substance abusers: one-year follow-up of the focus on families project. Addiction. 1999;94(2):241-254.
- Caplehorn JR, Dalton MS, Haldar F, et al. Methadone maintenance and addicts’ risk of fatal heroin overdose. Subst Use Misuse. 1996;31(2):177-196.
- McEvoy GK, Welsh OH, Snow EK, eds. American Hospital Formulary Service Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 1999.
- Salpeter SR, Buckley JS, Bruera E. The use of very-low-dose methadone for palliative pain control and the prevention of opioid hyperalgesia. J Palliat Med. 2013;16(6):616-622.
- Albutt C. On the abuse of hypodermic injections of morphia. Practitioner. 1870;5:327-331.
- Rossbach M. Ueber die Gewoehnung an Gifte. Pflugers Arch Gesamte Physiol Menschen. 1880;21:213-225.
- Ramirez-Cacho WA, Flores S, Schrader RM, et al. Effect of chronic maternal methadone therapy on intrapartum fetal heart rate patterns. J Soc Gynecol Investig. 2006;13(2):108-111.
- Tsai LC, Doan TJ. Breastfeeding among mothers on opioid maintenance treatment: a literature review. J Hum Lact.2016;32(3):521-529.
- Chana SK, Anand KJ. Can we use methadone for analgesia in neonates? Arch Dis Child Fetal Neonatal Ed. 2001;85(2):F79-F81.
- Vieweg WV, Hasnain M, Howland RH, et al. Methadone, QTc interval prolongation and torsade de pointes: Case reports offer the best understanding of this problem. Ther Adv Psychopharmacol. 2013;3(4):219-232.
- Martin JA, Campbell A, Killip T, et al. QT interval screening in methadone maintenance treatment: report of a SAMHSA expert panel. J Addict Dis. 2011;30(4):283-306.
- Ehret GB, Voide C, Gex-Fabry M, et al. Drug-induced long QT syndrome in injection drug users receiving methadone: high frequency in hospitalized patients and risk factors. Arch Intern Med. 2006;166(12):1280-1287.
- Lin C, Somberg T, Molnar J, et al. The effects of chiral isolates of methadone on the cardiac potassium channel IKr. Cardiology. 2009:113(1):59-65.
- Krantz MJ, Rowan SB, Mehler PS. Cocaine-related torsade de pointes in a methadone maintenance patient. J Addict Dis. 2005;24(1):53-60.
- Alinejad S, Kasemi T, Zamani N, et al. A systematic review of the cardiotoxicity of methadone. EXCLI J. 2015;14:577-600.
- John J, Amley X, Bombino G, et al. Torsade de pointes due to methadone use in a patient with HIV and hepatitis C coinfection. Cardiol Res Pract. 2010;524764.
- Chugh SS, Socoteanu C, Reinier K, et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
- Chou R, Cruciani RA, Fiellin DA, et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain. 2014;15(4):321-337.
- Esses JL, Rosman J, Do LT, et al. Successful transition to buprenorphine in a patient with methadone-induced torsades de pointes. J Interv Card Electrophysiol. 2008;23(2):117-119.
- de Jong IM, de Ruiter GS. Buprenorphine as a safe alternative to methadone in a patient with acquired long QT syndrome: a case report. Neth Heart J. 2013;21(5):249-252.
- Digby GC, Perez Riera AR, Barbosa Barros R, et al. Acquired long QT interval: a case series of multifactorial QT prolongation. Clin Cardiol. 2011;34(9):577-582.
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm. 2014;11(3):360-366.
- Miller MA, Bhasin K, Reddy VY, et al. Left cardiac sympathetic denervation for the treatment of methadone-induced long QT syndrome. Heart Rhythm. 2011;8(12):1955-1957.



Leave a Reply
You must be logged in to post a comment.