BACKGROUND
Administering the wrong medication is one of the most feared complications in any field of medicine. Anesthesia professionals are some of the only providers who prescribe, prepare, and administer their own medications. Therefore, the perceived fear among anesthesia professionals is even greater due to this unique responsibility. Medication error may occur for a variety of reasons. One of the most common sources of medication error is related to look-alike and sound-alike (LASA) drugs as well as the often-similar appearances of the vials. LASA medications are typically thought of as medications that are similar in physical appearance related to packaging as well as medications whose names are similar in spelling or in the phonetic pronunciation. This is a difficult problem to quantify because it is a moving target due to ever-shifting manufacturer trade names, new medications on the market, changes in packaging between different manufacturers, and the ever-changing formulary at individual hospitals. Further complicating the issue is that pharmacies must pivot frequently by changing who they order medications from as they manage frequent drug shortages. The sudden change in appearance of a medication vial that the team has previously grown accustomed to can be disruptive and lead to increased risk of a medication error.
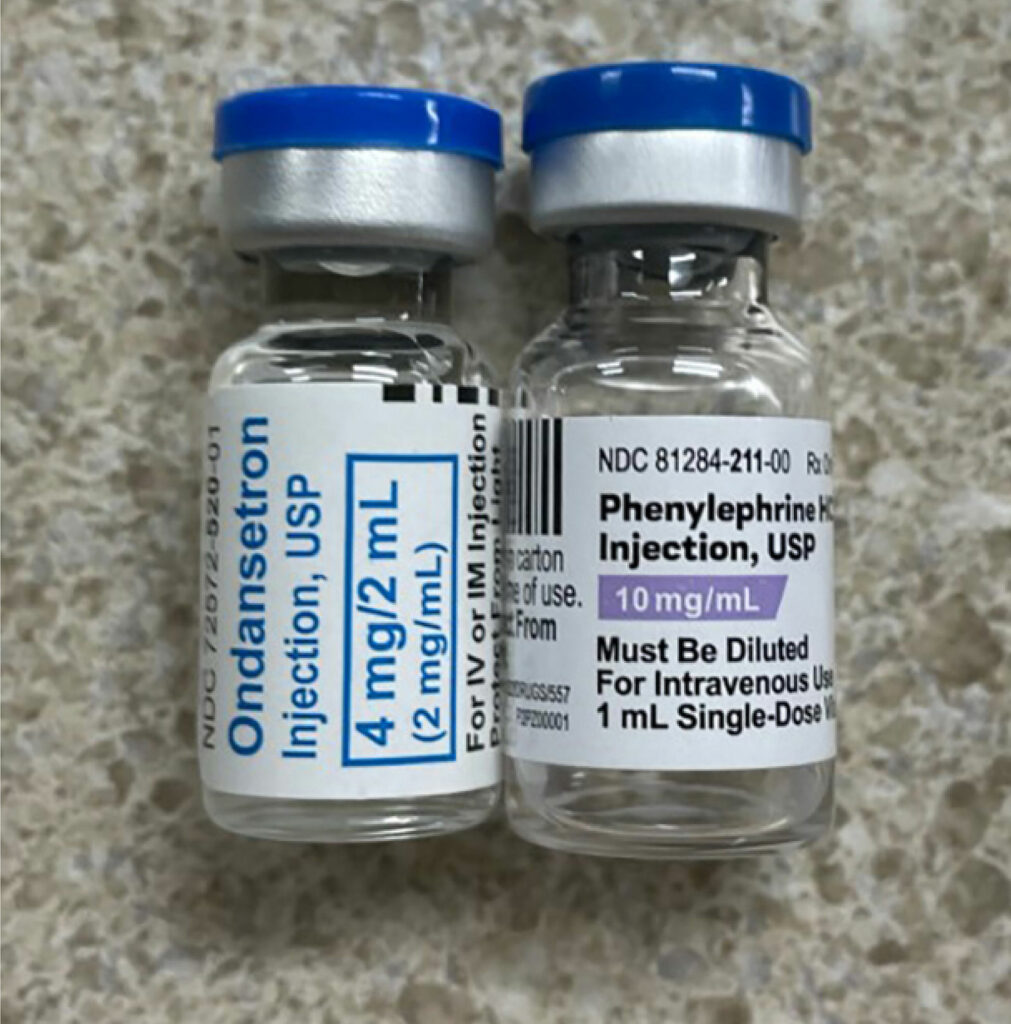
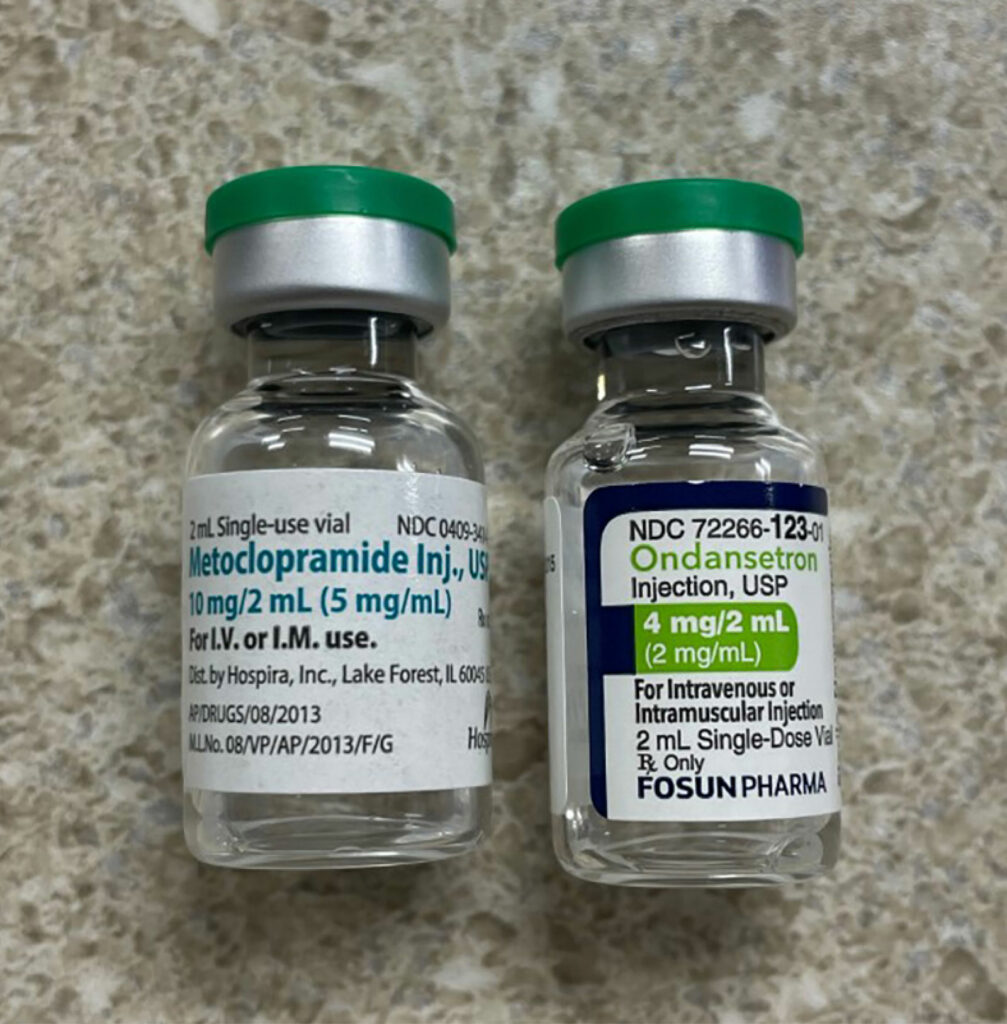
In a recently published article which reviewed the first 4000 incident reports in the webAIRS anesthetic incident reporting system from Australia and New Zealand anesthesia professionals, the authors found 462 incidents involved medication errors with incorrect dosing and substitution as the top-ranked error categories.1 A primary contributing factor for the substitution category were look-alike drugs.1 LASA-related mistakes are compounded when the involved medications are either high alert (e.g., opioids, insulin, anticoagulants, neuromuscular blocking agents, etc.) or hazardous (e.g., chemotherapy agents) or the route of administration is potentially dangerous (e.g., intrathecal). The issue is further compounded by the fact that each vial will have at least three names (chemical name, generic name [may vary by country], and often more than one brand or trade name). In addition, the medication vials can share many similarities in appearance such as color of the vial medication cap as well as similarities in the labels. (See Figures 1a, 1b, and 1c.)
INCIDENCE
It is difficult to know how many LASA errors occur, but it has been estimated that LASA errors account for as much as 25% of medication errors.2 Medication pairs that look-alike sound-alike may be one of the most common contributing factors to medication errors.3,4 Attempts by regulatory agencies, hospitals, and practitioners to eliminate these LASA errors have thus far been unsuccessful, and there are numerous recent examples in the literature and news.
CASES OF LASA ERRORS
There have been several very high-profile medication error cases in the recent past. The one that received the most attention recently occurred when a nurse intended to give a benzodiazepine (Midazolam [Versed]) to a patient to alleviate procedural anxiety. However, she entered the letters V-E into the automated medication dispensing cabinet (AMDC) and vecuronium was offered by the AMDC as the medication option to dispense and was chosen by the nurse. She bypassed several safety measures in order to withdraw and administer vecuronium to the patient, which led to the ultimate demise of the patient. The nurse was eventually tried and convicted of criminally negligent homicide. One of the primary issues was felt by many to be an unfamiliarity with the medications involved and the fact that multiple safety barriers were ignored in the process including warnings from the AMDC and on the medication vial’s cap and label.5
There have also been recent inadvertent administrations of the wrong medications intrathecally. Most notably, tranexamic acid and digoxin have been mistakenly administered into the subarachnoid space during attempted spinal block (Figure 2). These examples are attributed to the similar appearance of the ampules or vials for these medications. The mistaken administration of tranexamic acid intrathecally resulted in seizures and ventricular arrhythmias in the described cases.6-8
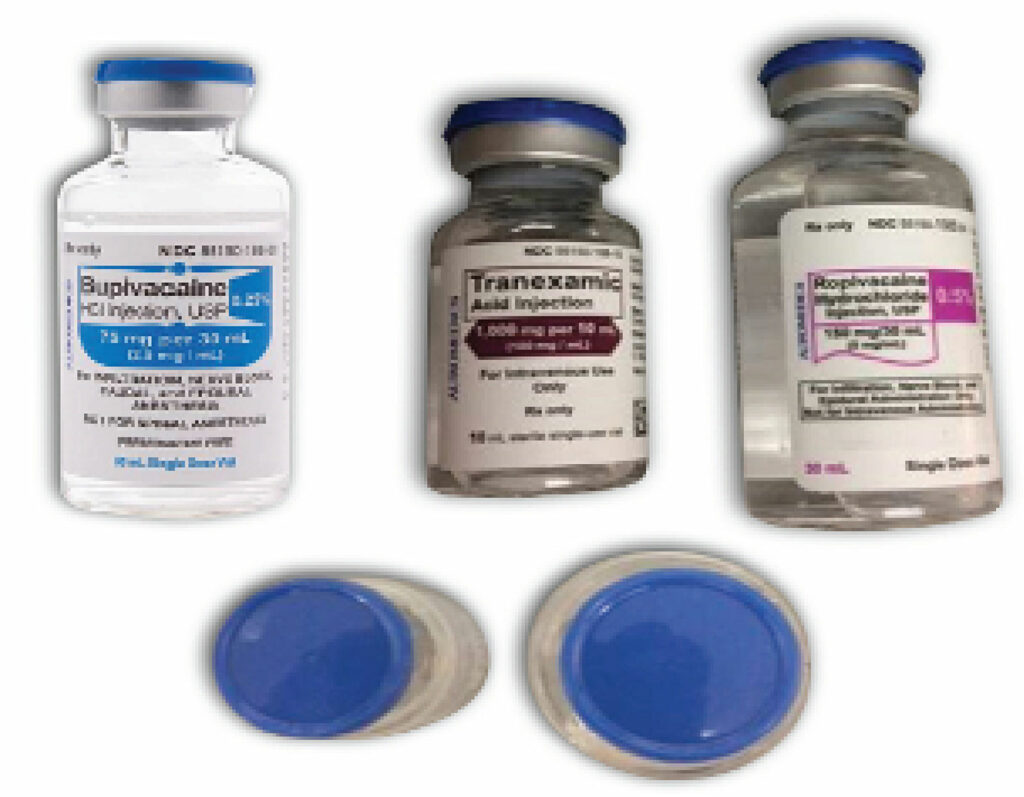
Figure 2: Look-alike vials of tranexamic acid, ropivacaine, and bupivacaine. While label colors and vial sizes are different, the caps are blue and if stored upright, may lead to selecting a vial based on cap color. (Used with permission from ISMP).8
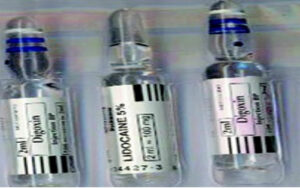
Figure 3: Look-alike vials of digoxin and lidocaine. (Used with permission from Anesthesia & Analgesia ).9
Intrathecal administration of digoxin has been associated with paraplegia and encephalopathy (Figure 3).9,10 A recent review of the literature found at least 8 incidences of accidental intrathecal injection of digoxin.10 Additionally, the review found a total of 33 instances of cardiovascular drugs accidentally administered via the neuraxial route often associated with devastating outcomes.10 In this review, incorrect visual inspection of look-alike ampules was found to be the most common factor in the mistaken administrations.
An additional two examples occurred in two separate instances when insulin was accidentally administered instead of an influenza vaccine in a group care facility and for an employee group. These incidents resulted in the hospitalization of multiple symptomatic individuals.11,12 Both of these instances were attributed to the similar appearances of the two vials.
PREVENTION TECHNIQUES
Regulatory agencies such as The Joint Commission (TJC) and the Food and Drug Administration (FDA) have identified these LASA errors as a focus in the past several years and have made efforts to eliminate them through education and tools to decrease the risk. The Joint Commission recommends that all hospitals have their own LASA list of medications. Instead of simply downloading one from the internet unchanged, they recommend that each site personalize the list to only include medications that are administered at the individual sites and utilize internal error reports associated with LASA drugs.13 They also recommend that the lists should be reviewed and updated at least annually.
In addition, the FDA has incorporated the “tall man lettering” (TML) system for drug names that may be confused due to similarities in appearance or sound.14 The TML system is a technique that utilizes uppercase lettering in a portion of the drug labeling where confusion may occur. For example, the written appearance of dexmedetomidine and dexamethasone are similar and could lead to confusion. Using TML, they would appear as dexmedeTOMidine and dexameTHASONE, drawing attention to the portions of the name which are dissimilar. Medications that receive this labeling modification are typically chosen because of similarities that occur in the spelling of the medication name, especially if these similarities have previously resulted in a reported drug error. The FDA also developed a computer analysis tool that measures the phonetic and orthographic similarities of the planned brand name of the medication against datasets from different sources including preexisting drug brand and generic names. The FDA’s intention is to assist in developing proprietary names for drugs that are less likely to cause errors.15 The American Society of Anesthesiologists adopted a statement on the labeling of pharmaceuticals for use in anesthesiology in 2004 and it was most recently updated in 2020.16 This document addresses the hazards of LASA drugs and includes a list of medications frequently used in anesthesiology which have been identified as high risk for LASA and the medication names are formatted using the TML system (Figure 4).
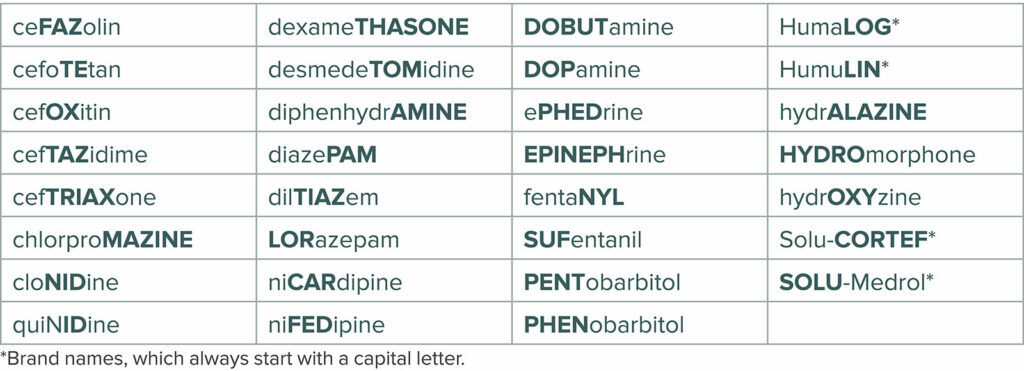
Figure 4: Tall Man Lettering of some drugs used in the perioperative setting. (https://www.asahq.org/standards-and-guidelines/statement-on-labeling-of-pharmaceuticals-for-use-in-anesthesiology ) (Reprinted with permission of the American Society of Anesthesiologists, 1061 American Lane, Schaumburg, Illinois 60173-4973).
Since 2008, the Institute for Safe Medication Practices (ISMP) has maintained a list of often confused drug names related to look-alike and sound-alike characteristics.17 However, due to lack of standardization in the packaging of medications, an additional list of medication that are similar in appearance in the packaging is difficult to compile.
Understanding that LASA medication errors can occur at every stage of the medication use process, ISMP and other groups have developed counter measures for each phase (procuring, prescribing/ordering, verifying, dispensing, administering, and stocking/storing).18 The administration phase may be the most vulnerable stage, as it is the least likely stage to catch an error.19,20 The following is a partial list with abbreviated strategies for problematic LASA medications from the Institute of Safe Medication Practices.18
PURCHASING
- Avoid stocking/purchasing medications in which the manufacturer’s trademark symbol/logo is larger than name of product.
- Ensure that names are evaluated by practitioners who use them before adding to formulary/inventory.
- Ask pharmacy to identify LASA concerns for medications that are new or shortage substitutes
ORDERING/PRESCRIBING
- Avoid abbreviations (e.g., MgSO4, TXA), stemmed, or stems (e.g.,“caines”), or shortened names (e.g., “dex”). Communicate the full generic name and/or brand name.
- Brand and generic name should be displayed for problematic look-alike names in the medication description field, on product selection menus, and for search choices
- Build order sets with the indications for problematic names (e.g., hydrOXYzine for pruritus, hydrALAZINE for hypertension).
ADMINISTRATION
- Before administering a medication, read the container and/or pharmacy label when obtaining from unit stock or AMDC. Never rely solely on a partially turned label, the color of a label/cap, the auxiliary warning, or company graphics to identify a product.
STOCKING/STORING
- In anesthesia carts/trays, organize vials in a label-up instead of cap-up position, and avoid close proximity with LASA names (or look-alike packaging and labeling, particularly cap colors).
NOMENCLATURE
- For problematic look-alike medication names, use tall man lettering on electronic prescribing drug selection screens, order sets, AMDC screens, smart infusion pump screens, medication administration records, and any other drug communication tools.
- If short names are permitted to search for products or populate fields without entering the full medication name, require practitioners to enter at least 5 letters during a drug name search to reduce the number of medications, including those with LASA names, that appear together on a screen.
(https://www.ismp.org/resources/adopt-strategies-manage-look-alike-andor-sound-alike-medication-name-mix-ups)
CONCLUSION
LASA medication errors have been described as a preventable threat to a patient safety. The oversight of the LASA drug dilemma is not just the responsibility of the frontline health care professional. There have been numerous strategies recommended, but there are multiple strategies for each of the stages of the medication use process and many are challenging to implement, particularly in a busy, fast-paced preoperative, intraoperative, and postoperative setting. Currently, there is little that can be done about existing drug names with LASA implications other than the suggested strategies. Health care professionals, safety groups, and professional organizations should continue to work with manufacturers, regulators, and naming entities to explore opportunities to minimize the LASA risks for drugs that are either new to the market or in the pre-marketing stage.15
REFERENCES
- Kim JY, Moore MR, Culwick MD, et al. Analysis of medication errors during anaesthesia in the first 4000 incidents reported to webAIRS. Anaesthesia and Intensive Care. 2022;50:204-219. PMID: 34871511
- Ciociano N, Bagnasco L. Look alike/sound alike drugs: a literature review on causes and solutions. Int J Clin Pharm. 2014;36:233–242. PMID: 24293334
- Wong ZSY. Statistical classification of drug incidents due to look-alike sound-alike mix-ups. Health Informatics J. 2016; 22:276–292 . PMID: 25391848
- McCoy LK. Look-alike, sound-alike drugs review: include look-alike packaging as an additional safety check. Joint Comm J Qual Patient Saf. 2005;31:47–53. PMID: 15691210
- Anesthesia Patient Safety Foundation (APSF). Position statement on criminalization of medical error and call for action to prevent patient harm from error. APSF Newsletter. 2022;37:1–3. https://www.apsf.org/article/position-statement-on-criminalization-of-medical-error-and-call-for-action-to-prevent-patient-harm-from-error/. Accessed March 31, 2023.
- Kaabachi O, Eddhif M, Rais K, Zaabar MA. Inadvertent intrathecal injection of tranexamic acid. Saudi J Anaesth. 2011;5:90–92. doi: 10.4103/1658-354X.76504. PMID: 21655027.
- Mahmoud K, Ammar A. Accidental intrathecal injection of tranexamic acid. Case Rep Anesthesiol. 2012;2012:646028. doi: 10.1155/2012/646028. Epub 2012 Mar 26. PMID: 22606407.
- Institute for Safe Medication Practices (ISMP). Dangerous errors with tranexamic acid. ISMP Medication Safety Alert! Acute Care. 2019;24:1–2. https://www.ismp.org/alerts/dangerous-wrong-route-errors-tranexamic-acid. Accessed March 17, 2023.
- Bagherpour A, Amri Maleh P, Saghebi R. Accidental intrathecal administration of digoxin. Anesthesia & Analgesia. 2006;103:502–503. PMID: 16861456
- Patel S. Cardiovascular drug administration errors during neuraxial anesthesia or analgesia—a narrative review. J Cardiothor Vasc Anesth. 2023;37:291–298. PMID: 36443173
- Watts A, Spells A. 10 hospitalized after insulin administered instead of flu shot. CNN. Updated Nov.8, 2019. https://www.cnn.com/2019/11/07/us/oklahoma-flu-shot-mix-up/index.html. Accessed March 12, 2023.
- Institute for Safe Medication Practices (ISMP). Fifty hospital employees given insulin instead of influenza vaccine. ISMP. May 5, 2016. https://www.ismp.org/resources/fifty-hospital-employees-given-insulin-instead-influenza-vaccine. Accessed March 20, 2023.
- Hunt B. Managing high-alert/hazardous and look-alike/sound-alike (LASA) medications in your Bureau of Primary Care Health Center. Dec. 3, 2019. https://www.jointcommission.org/-/media/tjc/documents/accred-and-cert/ahc/High_Alert_Hazardous_Look_Alike_Sound_Alike_Medications.pdf. Accessed March 17, 2023.
- Food & Drug Administration (FDA). FDA list of established drug names recommended to use tall man lettering (TML).FDA. April 28, 2020. https://www.fda.gov/drugs/medication-errors-related-cder-regulated-drug-products/fda-name-differentiation-project. Accessed March 17, 2023.
- Bryan R, Aronson JK, Williams A, Jordan S. The problem of look-alike, sound-alike name errors: drivers and solutions. Br J Clin Pharmacol. 2021;87:386–394. PMID: 32198938
- American Society of Anesthesiologists Committee on Equipment and Facilities. Statement of labeling of pharmaceuticals for use in anesthesiology. Update on Dec. 13, 2020. https://www.asahq.org/standards-and-guidelines/statement-on-labeling-of-pharmaceuticals-for-use-in-anesthesiology. Accessed March 17, 2023.
- Institute for Safe Medication Practices (ISMP). ISMP’s list of confused drug names. ISMP. Updated Feb. 2015. https://www.ismp.org/sites/default/files/attachments/2017-11/confuseddrugnames%2802.2015%29.pdf. Accessed March 12, 2023.
- Institute for Safe Medication Practices (ISMP). Adopt strategies to manage look-alike and/or sound-alike medication name mix-ups. ISMP Medication Safety Alert! Acute Care. June 2022;27:1–4. (https://www.ismp.org/resources/adopt-strategies-manage-look-alike-andor-sound-alike-medication-name-mix-ups). Accessed March 20, 2023.
- Austin J, Bane A, Gooder V, et al. Development of the Leapfrog Group’s bar code medication administration standard to address hospital inpatient medication safety. Journal of Patient Safety. 2022;18:526–530. PMID: 35797583
- Institute of Medicine, Committee on identifying and preventing medication errors. Aspden P, Wolcott J, Bootman JL, et al. editors. Washington, DC: National Academies Press (US); 2007. Available at: https://nap.nationalacademies.org/catalog/11623/preventing-medication-errors. Accessed March 21, 2023.


Leave a Reply
You must be logged in to post a comment.