Authors: Alanzi A K, et al
Cureus 15(4): e37351. doi:10.7759/cureus.37351
Abstract
An abdominal aortic aneurysm (AAA) is a disease characterized by an abnormal bulge or swelling in the aorta. It could be serious if left unobserved, and with time, it swells and eventually ruptures, resulting in massive bleeding inside, and, more likely, causes death. This report presents a case study of a 61-year-old male who presented with back pain; no other fatal symptoms such as breathlessness or rapid heart rate were seen. His abdominal ultrasound report showed the presence of a distal aortic dissecting aneurysm, resulting in rapid diagnosis and treatment.
Introduction
An abdominal aortic aneurysm (AAA) is a segmental, full-thickness dilation of the abdominal aorta that is 50% greater than the normal aortic diameter or abdominal aortic diameter, greater than 3 cm. In general, AAA tends to appear in males more than in females [1]. The diameter varies in the significance of clinical events depending on gender; in males, diameters are mostly predictive of clinical events of the aneurysm, whereas in females, it is more dependent on the aortic size index (ASI), calculated as diameter (cm)/body surface area (m2) [2]. AAA is found in 5%-10% of males aged 65-79 [3]. Moreover, the estimated prevalence of AAA in developed countries is 2%-8%, which is higher in males than in females (4%-8% versus 1%-1.3%) [2].
Growth and rupture risk of the aneurysm is associated with female gender, smoking, and chronic lung disease [4]. Elective repair is recommended for aneurysms discovered to be larger than 5.5 cm to prevent rupture [3]. There is interest in population screening to detect, monitor, and repair abdominal aortic aneurysms before rupture [3]. AAA repair can be endovascular or with open surgical repair (OSR) [5], depending on the availability of endovascular repair and surgical team preference.
Case Presentation
A 61-year-old male, with a known case of hypertension, dyslipidemia, and diverticulosis diagnosed more than 20 years ago, presented to the emergency department (ED) with a one-day history of left-sided abdominal pain, which started in the morning. According to the patient, the pain started at 11 am over the left lower back and radiated to the flank and then localized to the left iliac fossa and suprapubic region. His condition was not associated with nausea or vomiting and tolerated oral intake. The patient’s bowel was opened that morning at 10:30 am, with no per rectal (PR) bleed, no urinary symptoms, no fever, and no chills or rigors. Severe pain is similar to the previous attacks of his diverticulitis. His past surgical history includes vasectomy and colonoscopy done with polypectomy. He reported smoking a cigar and consuming approximately one bottle of wine five times per week. On arrival to the ED, the patient’s temperature was 36.7°C, respiratory rate (RR) was 20 breaths per minute (bpm), random blood glucose was 8 mmol, blood pressure (BP) was 80/50 mmHg, heart rate (HR) was 62 beats per minute (bpm), and oxygen saturation (SpO2) was 97%. On examination, his abdomen appeared soft and lax, with tenderness over the left lower abdomen. The requested ECG showed sinus bradycardia with an HR of 48 bpm. Table 1 shows the results of the venous blood gas test.
At first, he received 1 L of normal saline, and his blood pressure increased to 110/80 mmHg in ED; then, he received paracetamol, diclofenac, fentanyl 50 mcg, and morphine 3 mg. Another 500 ml of normal saline was given. The results from the laboratory initially are showed in Table 2.
He was referred to general surgery with the impression of diverticulitis and sepsis.
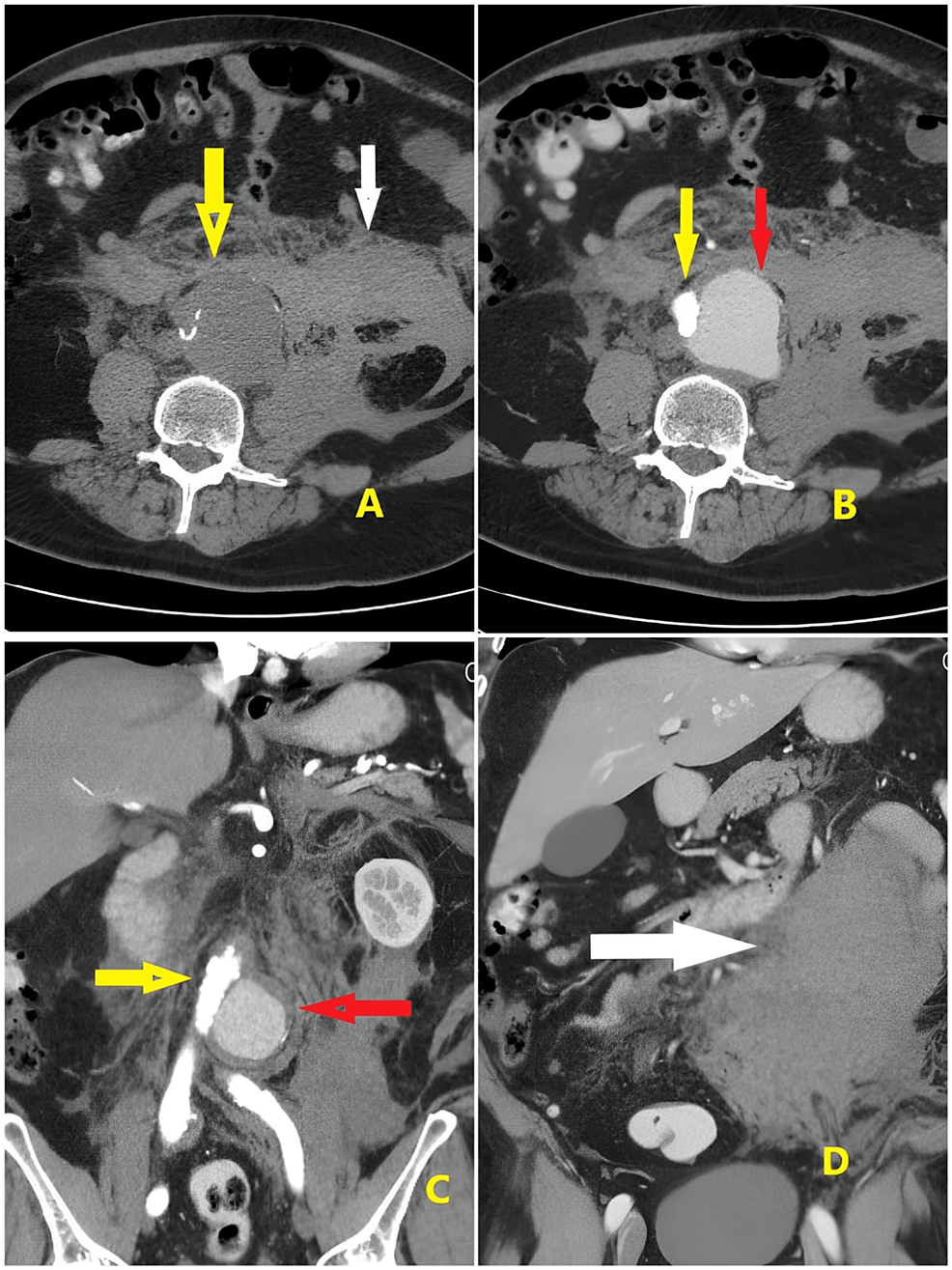
After the examination, the emergency team planned for computed tomography (CT) of the abdomen, but suddenly, his BP dropped to 60/30 mmHg. He was resuscitated in the ED, and 0.05 mcg/kg norepinephrine was started. Gradually, his BP rose to 100/60 mmHg. His HR was 54 bpm, BP was 102/60 mmHg, RR was 17 bpm, temperature was 36.6°C, and SpO2 was 99. The patient’s abdomen was re-examined, and tenderness was noted on the left side. Bilateral distal pulses were intact in the lower limbs. The focused assessment with sonography for trauma (FAST) scan showed free fluid in the abdomen. The abdominal X-ray report showed no free air and no air fluid. Phleboliths are noted on the pelvis. An abdominal ultrasound was performed, which revealed an infrarenal ruptured aortic aneurysm with a retroperitoneal bleed. Six units of packed red blood cell (PRBC), six units of platelets, and six units of fresh frozen plasma (FFP) were brought. Abdominal CT report reveals a distal aortic dissecting aneurysm involving the aortic bifurcation and extending to the origin of the left common iliac artery, measuring about 6 cm in maximum length and 6.2 cm in maximum cross-sectional diameter; the true lumen was seen attenuated and compressed to the right side (Figure 1B, yellow arrow), and the false lumen was dilated and faintly filled with contrast at the arterial phase (Figure 1B, red arrow) with more filling at the venous phase measuring about 5.5 × 4.5 cm in maximum axial diameter.
The anterior margin of the psoas muscle is obscured by a blood collection (Figure 1A, 1B, 1D, white arrow). Multiple colonic diverticula were seen more numerous at the sigmoid colon with no acute diverticulitis and patent homogeneously enhanced portal vein and its main divisions. The spleen was average in size, showing normal texture. Both kidneys were of normal size, showing good excretory function; the pancreas was of normal CT appearance and the suprarenal glands and inferior vena cava (IVC). Normal urinary bladder filling showed no stones, masses, or diverticula-enlarged prostate indenting the bladder base. No evidence of enlarged retro-crural, porta-hepatis, or para-aortic lymph nodes was noted.
Lower chest cuts revealed mild bilateral pleural reaction more on the left side. The patient was shifted to the theater at 17:30 for emergency AAA rupture repair, and on arrival to operation theater (OT), the patient had an HR of 115 bpm, BP of 95/50 mmHg, and oxygen saturation of 99%. The patient was intubated with rapid sequence induction and ventilated on pressure-control volume-guaranteed mode. The right internal jugular central line was inserted, 20 G right radial arterial line and 18 G left external jugular line. Right radial arterial line was done after Allen’s test, and right jugular central line was done with ultrasound guidance. Etomidate 18 mg and succinylcholine 100 mg were given. After intubation, the patient received atracurium 50 mg. During the procedure, the patient received boluses of epinephrine 100 mcg of a total of 900 mcg, a norepinephrine infusion of 4 mg in 50 ml Ringer’s lactate at a rate of 7-24 ml/hour, sodium bicarbonate 100 ml, and unfractionated heparin 5000 IU. Arterial blood gas (ABG) showed a pH of 7.173, partial pressure of carbon dioxide (PCO2) of 42, bicarbonate (HCO3) of 15.6, hemoglobin (Hb) of 6.1, potassium (K) of 4.9, and sodium (Na) of 139. Aortic clamping was done at 20:15; clamping time was approximately two hours. Repeated ABG showed pH of 7.19, Hb of 9.3, PCO2 of 38, HCO3 of 14.4, K of 4.4, and Na of 137. Total blood loss was 6 L, with total urine output of 500 ml. The input was 8 L of crystalloids, six packed red blood cells (RBCs), and four units of FFP.
The patient was shifted to the intensive care unit (ICU) after repair with an interposition graft. He was first intubated and then sedated on propofol and remifentanil-controlled mandatory ventilation (CMV); his tidal volume (TV) was 450 ml, respiratory rate was 22 bpm, positive end expiratory pressure (PEEP) was 5 cmH2O, fraction of inspired oxygen (FiO2) was 100%, and BP was 60/40 mmHg on norepinephrine 0.3 mcg/kg/minute. The patient was shifted to OT the next day for revision laparotomy for leaking venous blood and ischemic transverse colon with transverse colectomy and loop colostomy. He was intubated and ventilated, his BP was 56/40 mmHg, he was on noradrenaline infusion of 1 mcg/minute, his SpO2 was 88%, and his FiO2 was 90%. The patient was sedated with remifentanil 15 mcg/minute and propofol 60 mg/hour, with synchronized intermittent mandatory ventilation (SIMV)-pressure support (PS) mode of ventilation. The patient initially received a noradrenaline infusion of 1 mcg/kg/minute, which was later changed to adrenaline 0.2 mcg/kg/minute as norepinephrine did not respond. Adrenaline 100 mcg bolus was given intermittently in between. The total blood loss was 500 ml, and the patient was anuric. ABG test results are shown in Table 3.
At the end of the procedure, he was shifted back to the ICU and was intubated and ventilated. His BP was 93/49 mmHg, pulse rate (PR) 90/minute, and SpO2 96% with adrenaline infusion of 0.2 mcg/kg/minute. He developed acute ischemic hepatitis, acute kidney injury, and disseminated intravascular coagulation (DIC). Two days later, his left foot showed bluish discoloration and was cold to touch with absent dorsalis pedis pulsation. And transverse colostomy was malfunctioning. The next day, he was taken to OT for relook laparotomy to explore and revise the rectal stump and left femoral embolectomy by vascular surgery.
He was shifted to OT with unstable hemodynamics. He was given a dopamine supramaximal dose; his SpO2 was 85% and FiO2 100% and was put on ventilator. He was started on a noradrenaline infusion of 4 mg in 50 ml at 2-10 ml/hour. The patient was arrested intraoperatively and was resuscitated. Spontaneous circulation returned after three cycles of cardiopulmonary resuscitation (CPR), two doses of adrenaline, and direct cardioversion twice. Ten minutes later, the patient had ventricular tachycardia and received lignocaine 140 mg; synchronized cardioversion was done with 150 J. Sodium bicarbonate 75 ml was given. He received two units of PRBC and was admitted to the ICU. Acute kidney injury on continuous renal replacement therapy (CRRT) disseminated intravascular coagulation. The patient was intubated and sedated for one week with frequent weaning off and reintubation for another week and then extubated. He was shifted to the ward on the June 10, 2020, and was vitally stable on hemodialysis. The patient was discharged after two weeks.
Discussion
A ruptured abdominal aortic aneurysm request is one of the “hottest” cases on our CT scanner regarding the need for speed. The mortality rate is very high: >90% [6]. Hypertension is considered a potential risk factor that leads toward AAA [7]. Abdominal aortic aneurysm rupture is a surgical emergency. The treatment of an acute rupture should be prompt and can be done with endovascular aneurysm repair or open surgery. It was noted from the retrospective data from the United States that over the past decade, the use of open surgical repair (OSR) has decreased, and a notable increase is observed in the use of endovascular repair for ruptured AAAs [8]. The mortality after rupture is high, 80% for patients reaching the hospital and 50% for those undergoing surgery for emergency repair [2]. In this case, the emergency department team, general and vascular surgeons, anesthetists, and the ICU team resuscitated the patient and provided optimum care. Table 4 shows some similar cases of AAA.
Conclusions
Pain is one of the most common symptoms found in patients suffering from AAA, but unfortunately, this is very common, so most people do not seem concerned about it, and before they know about the consequences, it is too late. People should be aware of the importance of screening programs. We suggest that everyone have regular clinical visits and checkups to minimize the chances of such life-threatening events.
References
- Kumar Y, Hooda K, Li S, Goyal P, Gupta N, Adeb M: Abdominal aortic aneurysm: pictorial review of common appearances and complications. Ann Transl Med. 2017, 5:256. 10.21037/atm.2017.04.32
- Dalman RL, Mell M: Overview of abdominal aortic aneurysm. UpToDate. UpToDate, Waltham, MA; 2021.
- Cosford PA, Leng GC: Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007, CD002945. 10.1002/14651858.CD002945.pub2
- Ullery BW, Hallett RL, Fleischmann D: Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom Radiol (NY). 2018, 43:1032-43. 10.1007/s00261-017-1450-7
- Kyriacou H, Mostafa AM, Sumal AS, Hellawell HN, Boyle JR: Abdominal aortic aneurysms part two: surgical management, postoperative complications and surveillance. J Perioper Pract. 2021, 31:319-25. 10.1177/1750458920947352
- Hou Y, Guo W, Fan T, Li B, Ge W, Gao R, Wang J: Advanced research of abdominal aortic aneurysms on metabolism. Front Cardiovasc Med. 2021, 8:630269. 10.3389/fcvm.2021.630269
- Kobeissi E, Hibino M, Pan H, Aune D: Blood pressure, hypertension and the risk of abdominal aortic aneurysms: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2019, 34:547-55. 10.1007/s10654-019-00510-9
- Greco G, Egorova N, Anderson PL, et al.: Outcomes of endovascular treatment of ruptured abdominal aortic aneurysms. J Vasc Surg. 2006, 43:453-9. 10.1016/j.jvs.2005.11.024
- Petriceks AH, Olivas JC, Salmi D: Educational case: symptomatic but unruptured abdominal aortic aneurysm. Acad Pathol. 2018, 5:2374289518798560. 10.1177/2374289518798560
- Clancy K, Wong J, Spicher A: Abdominal aortic aneurysm: a case report and literature review. Perm J. 2019, 23:18.218. 10.7812/TPP/18.218
- Garrity BM, Sugarman E, Pulley S: Abdominal aortic aneurysm rupture presenting with focal weakness and altered mental status: a case report. Int J Emerg Med. 2022, 15:28. 10.1186/s12245-022-00433-5


Leave a Reply
You must be logged in to post a comment.