Anesthesiology News
Anesthesia providers are often confronted with the management of a “cuff leak” in mechanically ventilated patients in the OR and ICU.1 Certainly, the advantage of the OR environment is that the patient is in a relatively more controlled setting, monitored by health care providers (HCPs) who are familiar with the patient’s airway and positioning.
An “air leak” in the ICU is often more challenging because of its remote location. Being summoned to a remote location presents unique issues. The acutely ill ICU patient is likely unknown to the airway team. Moreover, a plethora of conditions mark the mechanically ventilated ICU patient in an elevated risk category compared with most elective surgical patients. Suboptimal or limited airway visualization due to an indwelling endotracheal tube (ETT), secretions, edema, limited oral access, cervical spine appliances, the presence of orogastric feeding tubes, anatomic constraints and restricted positioning abilities are just a few of the challenging conditions that make the ICU patient unique.1-6
The characteristics of the leak are variable. The leaking air may be continuous or intermittent and may vary with the patient’s overall body positioning as well as the specific positioning of the head and neck. The air leak may compromise ventilation and oxygenation plus expose the patient to the risks for aspiration of gastric contents and soilage from oronasal secretions.
Simply assuming that a cuff leak is related strictly to the cuff itself is a common mistake. The broad differential diagnoses for the air leak demand an understanding of their variable etiologies so that the correct reparative measure can be applied. As will be discussed later, there is the potential for grave consequences if the incorrect management schema is pursued.
A cuff leak may be caused by a structural defect in the cuff (i.e., perforation) or a dysfunctional pilot balloon and inflation valve system. However, as outlined by El-Orbany and Salem, the etiology of an air leak is much more varied and extensive.1 An air leak may be caused by cuff underinflation, cephalad migration of the ETT (partial tracheal extubation), misplaced orogastric or nasogastric tubes, a discrepancy between ETT and tracheal diameters, or increased peak airway pressure causing a leak around an intact cuff (Table 1). An understanding of this broad differential will allow the provider to understand the variable corrective measures required to eliminate the cuff leak without having to replace the ETT (Table 2). Applying an inappropriate corrective measure may lead to dire and life-threatening consequences.1-4,7
| Table 1. Differential Diagnosis Of a Cuff Leak |
| ETT cuff (within trachea) |
|---|
|
|
|
|
|
| ETT cuff (above trachea) |
| ETT cuff–tip complex dislocation (partial/complete extubation) |
|
|
|
|
a For example, nasogastric tube, orogastric tube, temperature probe, tooth.
b Tracheoesophageal, gastrotracheal, gastrobronchial, bronchoesophageal fistula.
ETT, endotracheal tube
|
| Table 2. Simplified Problem–Solution Table for a Cuff Leak | |
| Problem | Solution |
|---|---|
| Tracheo-carinal/main stem intubation | Reposition ETT |
| TE fistula (gastro-, esophago-, broncho-) | Surgical consult, reposition ETT, radiographic studies |
| Cuff perforation | Change the ETT |
| Incompetent valve/pilot balloon line | Repair the valve/clamp line/exchange ETT |
| Tracheal wall–cuff incongruity | Advance/retract/change size/remove NGT |
| Partial/complete tracheal extubation | Fiber-optic bronchoscopy or DL/VL assessment, and resecure the airway |
| DL, direct laryngoscopy; ETT, endotracheal tube; NGT, nasogastric tube; TE, tracheoesophageal; VL, video laryngoscopy | |
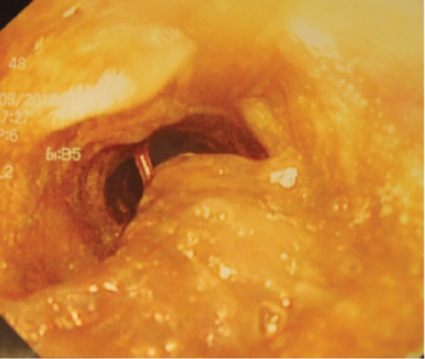
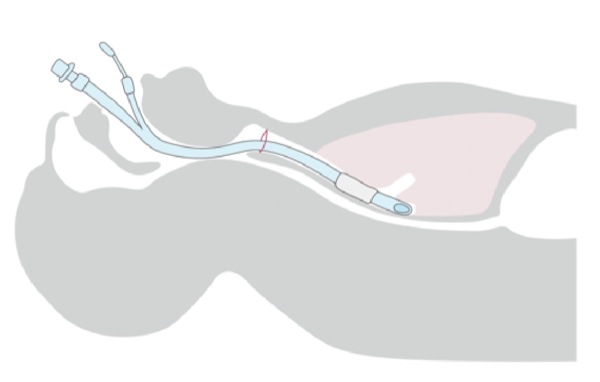
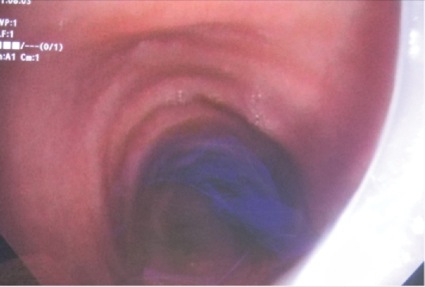
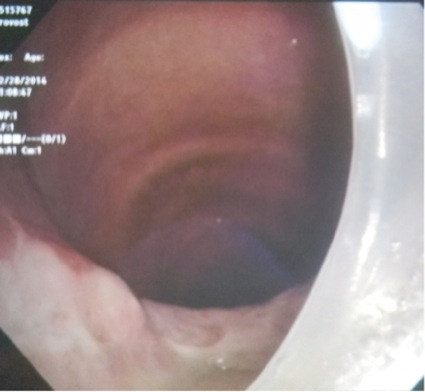
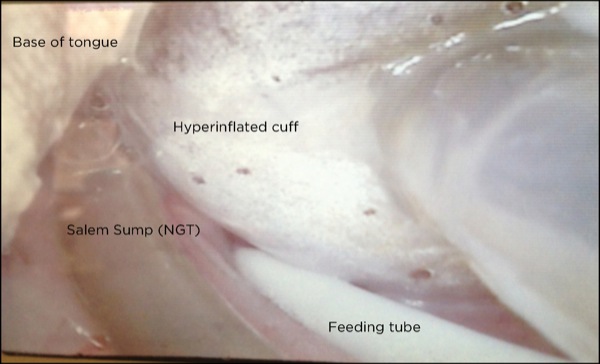
Cephalad migration of the ETT cuff and tip leading to unrecognized partial or complete extubation may be the most common cause of an air leak not related to the cuff itself.1,5,7 For more precise communication, understanding and ease of discriminating between the various levels of ETT dislocation among HCPs, a grading system to define the various ETT tip–cuff positions may be appropriate. Figure 1 shows an ETT positioned correctly in the trachea. Partial or complete extubations can be classified by the location of the ETT cuff and tip:
- In grade I, the cuff is between the vocal cords (Figure 2).
- In grade II, the cuff is above the vocal cords with the tip at the level of the vocal cords/arytenoids (Figure 3).
- In grade III, the tip–cuff complex completely above the glottic opening and within the hypopharynx (Figure 4).
The diagnosis of partial or complete extubation is often delayed or unrecognized for a number of reasons. The perceived air leak is often managed by repeated additions of air to the pilot balloon. This may temporarily resolve the leak but result in further ETT displacement (a grade I or II extubation becoming a grade III complete extubation). Once the cuff has migrated between the vocal cords and a leak is appreciated, additional cuff air will only expand the cuff and typically force it to follow the path of least resistance (upward) due to patient head/neck movement, swallowing, coughing, and repositioning (e.g., during bedside chest radiography). Surprisingly, clinical signs and symptoms of partial or complete tracheal extubation in a sedated ICU patient may be discreet and delay recognition by HCPs.
Cuff Leak Etiologies
To explore patient safety concerns, management schema, complications and other clinical factors regarding partial and complete mostly unrecognized tracheal extubations, the Hartford Hospital emergency airway management database was explored. Overall, a total of 1,460 mechanically ventilated patients with a cuff leak were documented in the database (years 2005-2018) and underwent assessment by the anesthesia airway team.
The patients were cared for in one of five ICU locations within a Level 1 trauma center. The institutional review board (IRB) approved a retrospective review (with a waiver of consent) of the ETT exchanges and partial or complete extubations to determine clinical factors, management issues, airway evaluations and complications.
A laryngoscopic assessment of each patient’s airway was commonly performed with conventional direct laryngoscopy (DL) during the early years of the study period. When available in late 2006, video laryngoscopy (VL) with the GlideScope GVL (reusable blade, 2006-2009), GlideScope Cobalt (disposable blade, 2009-2018) and GlideScope Titanium (disposable blade, 2018; all from Verathon) became the method of choice.
A flexible fiber-optic bronchoscope (FFB) was available in all ICU units on the difficult airway cart for urgent/emergent use, in accordance with national and international recommendations.8-10Conversely, a bronchoscopic tower (fiber-optic tower/workstation) could be brought to the bedside by the respiratory therapy staff for semi-elective interventions. Bronchoscopy was frequently used to assist the team in determining the etiology of the cuff leak.
The anesthesia airway team consisted of a board-certified attending anesthesiologist either alone (22%) or more commonly supervising an anesthesia resident trainee (78%). During daytime hours during the weekdays (7 a.m. to 5 p.m.), the anesthesia intensivist on duty commonly oversaw all airway procedures. The anesthesiologist on call was responsible for all other coverage. All patients were administered 100% oxygen and positioned, if applicable, to optimize airway management (e.g., by ramping). The vast majority of patients (>90%) were receiving concurrent parenteral sedative-analgesic agents for mechanical ventilation. Laryngoscopy conditions were optimized by pharmacologic means with additional administration of IV propofol (1-2.5 mg/kg) or etomidate (0.1-0.3 mg/kg), with supplemental local anesthesia agents (lidocaine) instilled via the ETT (bronchoscopy) or by topical administration to the oral cavity/oropharynx, as needed. Neuromuscular blocking agents were not typically administered for the airway evaluation/intervention. This was particularly true when partial or complete tracheal extubation was deemed the most likely etiology for the cuff leak.
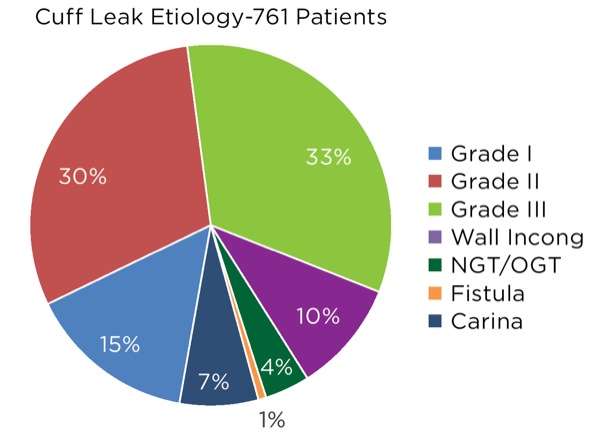
Of the 1,460 patient encounters in the database identified as an “evaluation for a cuff leak,” airway examination revealed that 699 patients proved to have a properly positioned ETT accompanied with a cuff perforation, an incompetent valve/pilot balloon, line assembly dysfunction or leak, or ETT damage (e.g., damage to the ETT wall from biting). These patients underwent ETT exchange. As presented in Table 1, the remaining 761 patients had a variety of etiologies contributing to the cuff leak. The majority of cuff leak evaluations uncovered previously unrecognized partial or complete tracheal extubation events (595/761, 78.2%; Figure 5 and Case 1).
The next most common cause was ETT–trachea wall incongruity that led to a tracheal wall–ETT cuff gap accounting for the cuff-related air leak (n=76, 10%; Case 2).1 This malady was associated with a variety of factors that led to a gap between the cuff and the tracheal wall. Specifically, these factors include the cuff design and shape, ETT caliber, instances when the ETT cuff was abutting against the underside of the glottic opening, anatomic tracheal wall distortions, inadequate cuff inflation volume/pressure, and tracheitis or tracheomalacia.
Although categorized as a separate entity for contributing to a cuff leak, any foreign body or medical device positioned between the ETT cuff and the tracheal wall could essentially contribute to a tracheal wall–ETT cuff gap defect. The presence of an intratracheal temperature probe, orogastric tube, nasogastric tube, enteral feeding tube or a foreign body (e.g., a tooth) accounted for a small portion of cases (3.9%; Case 2).
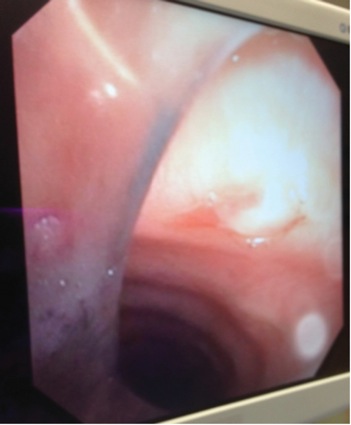
A main stem bronchial intubation with both the tip and cuff within the bronchus would not typically render a cuff leak. However, an ETT cuff positioned adjacent to or at the widening portion of the tracheo-carinal junction was documented fairly frequently (n=53, 7%; Figure 6 and Case 3).
Lastly, a rarely observed etiology was a communication (fistula, erosion or perforation) between the trachea or bronchus with the upper alimentary tract. The five instances were each related to previous complex surgical reconstruction procedures related to the esophagogastric structures (0.7%; Case 4).
Importance of Investigating the Leak
Due to the variety of cuff leak etiologies, it is imperative to investigate the leak rather than assume that the ETT cuff is leaking or dysfunctional. Determining the cause of the cuff leak will assist in planning corrective measures, if indicated. Thus, an airway assessment and review of the patient’s history and recent bedside activities—for example, recent placement of a feeding tube—may offer valuable information to assist the airway team in the delivery of optimal patient care.
For example, the airway team may be asked to “change the ETT” due to a supposed cuff leak. If the team assumes these are facts, they may simply advance an airway exchange catheter (AEC) via the existing ETT, remove it, and advance a new ETT over the indwelling AEC, while not realizing the original ETT was actually dislocated in the hypopharynx (herniation above the glottis). The newly replaced ETT may now be located outside the tracheal airway. This illustrates the importance of a preexchange or preprocedural airway assessment. Moreover, if the team decided to administer a neuromuscular blocking agent for the exchange procedure, this may have a detrimental impact given the unrecognized tracheal extubation.
Table 2 presents a streamlined problem–solution outline. Each etiology of the cuff leak may have a differing corrective measure that could endanger the patient if implemented erroneously. Performing an external airway assessment complemented by FFB and/or laryngoscopy (conventional DL or, preferably, VL) will allow the team to better define the problem and then develop a management plan.
Partial and Complete Tracheal Extubations
Proximal or cephalad migration of the ETT cuff and tip leading to unrecognized partial or complete extubation may occur acutely or incrementally over time.1 An air leak is often managed by repeated additions of air volume to the pilot balloon over hours or shifts because the HCP believes there is a cuff leak. This can result in further ETT displacement (a grade I/II extubation becoming a grade III complete extubation due to continued cephalad migration).
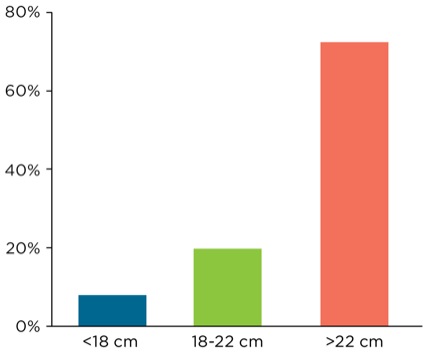
One keen observation gleaned from this database is that, in the presence of a cuff leak, the depth marking of the ETT may have little correlation to the actual ETT tip depth. Specifically, by examining the 595 encounters of ETT displacement, it was evident that the ETT depth, as noted at the dental/gingival line, was notoriously misleading as to the true depth or location of the ETT tip–cuff complex (Figure 7 and Table 3). Intuitively, an ETT noted at <18 cm depth at the gingival line would strongly suggest proximal ETT migration, yet overall, only 7.9% of the 595 displaced ETTs were documented as being less than 18 cm at the gingival line.
| Table 3. Depth of ETT as Noted At the Dentition/Gingival Line | |||
| Gradea | <18 cm Depth | 18-22 cm Depth | >22 cm Depth |
|---|---|---|---|
| I | 6% | 16.2% | 77.8% |
| II | 4.9% | 21.6% | 73.5% |
| III | 11.5% | 19.8% | 68.7% |
| Total | 7.9% | 19.7% | 72.4% |
|
a Grade of partial or complete tracheal extubation.
ETT, endotracheal tube |
|||
Further, only one in five encounters (19.7%) were noted at a depth of 18 to 22 cm. Finally, nearly three of every four patients had their ETT secured at a depth greater than 22 cm at the dentition/gingival line (72.4%). A minority of the displaced ETTs were shallow (<18 cm) regardless of grade of tracheal extubation (Table 3). Thus, ETTs that appeared to be at a sufficient depth may prove to be proximally displaced due to cephalad migration and cuff herniation supraglottically. It would appear that the purported depth is neither reassuring nor accurate in assisting the airway team or the ICU HCPs in determining the actual location of the ETT tip–cuff complex. Of note, checking the ETT depth at the lips/dentition line is only a single and often inaccurate factor in the airway assessment of a patient with a cuff leak.
Resecuring the Airway in Partial And Complete Tracheal Extubations
All cases (diagnostic and therapeutic) were managed in a nonrandomized fashion based on the judgment and skills of the airway team. Resecuring the airway was approached with a variety of methods, although the majority were via FFB or VL interventions (Table 4).1 Overall, VL assisted the airway team with resecuring the airway, for either elective use or rescue, in 211 of the 595 encounters (35.5%). An obstructed FFB view due to secretions/abutment against airway structures or difficult FFB/ETT advancement led to VL-assisted rescue in 21 cases. Although management was random and noncontrolled, VL use was more common in grade I and II cases, whereas FFB use was considered the primary method mainly for grade II and III cases (Table 4). Of note, many events categorized as FFB actually had a VL assessment performed initially. For example, a grade III extubation was uncovered during VL airway assessment. The airway team deployed bronchoscopy in a therapeutic manner to resecure the airway. Utilization of DL for the encounters fell sharply following the introduction and ubiquitous availability of VL.
| Table 4. Partial and Complete Tracheal Extubation: Initial Management (N=595) | |||
| Method/Device | Grade I (n=117) | Grade II (n=226) | Grade III (n=252) |
|---|---|---|---|
| DL (n=69, 11.6%) | 25 | 29 | 15 |
| VL (n=170, 28.6%) | 50 | 84 | 36 |
| AEC (n=18, 3.0%) | — | 6 | 12 |
| VL + AEC (n=15, 2.5%) | 1 | 10 | 4 |
| FFB (n=323, 54.3%) | 38 | 97 | 188 |
| AEC, airway exchange catheter; DL, direct laryngoscopy; FFB, flexible fiber-optic bronchoscopy; VL, video laryngoscopy | |||
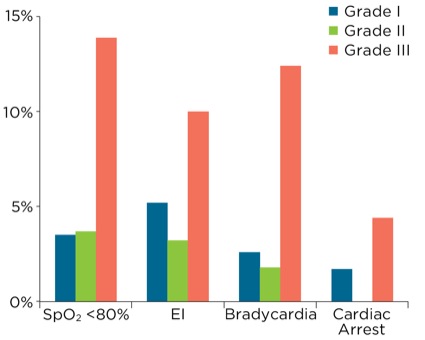
The trachea was resecured in grade I and II cases with a low incidence of complications. However, in the few cases in which complications occurred, the results were serious and life-threatening (Table 5). Figures 8 to 11 depict views of grade I and II ETT displacements via VL or bronchoscopic views. Despite the location of the ETT tip and cuff being in close proximity to the glottis, complications were related to advancing the existing, nonstyleted thermolabile (softened) ETT during reintubation attempts. Tracheal tip deflection into the esophagus, kinking of the softened ETT during advancement, and the presence of airway edema/secretions contributed to difficulty during laryngoscopy (both DL and VL).
Grade III extubation presented a much lower margin of safety and was fraught with life-threatening reintubation complications. The proximal migration of the ETT tip coupled with ETT stabilization at the lip/dentition line commonly culminated in an S-shaped (serpentine) curvature or bowing/arching ETT configuration. This could be appreciated with both FFB and VL assessment. The serpentine curvature led directly to the laterally directed ETT tip impinging against the airway wall/tissues. In the cases where the ETT tip abutted against the tracheal wall, FFB-assisted ETT advancement was made by retracting the ETT proximally away from the impingement, followed by FFB advancement distally into the lower trachea. This maneuver helped in many cases, but also commonly proved difficult (e.g., secretions) and failed.
| Table 5. Complications by ETT Location: Resecuring the Airway | |||
| Complication | Grade I (n=117) | Grade II (n=226) | Grade III (n=252) |
|---|---|---|---|
| Hypoxemia (<90%) | 6.0% | 4.6% | 13.1% |
| Hypoxemia (<80%) | 3.5% | 3.7% | 13.9% |
| Esophageal intubation | 5.2% | 3.2% | 10.0% |
| Bradycardia | 2.6% | 1.8% | 12.4% |
| Cardiopulmonary arrest | 1.7% | — | 4.4% |
| 3+ or more attempts | 2.6% | 2.2% | 11.9% |
| Rescue device requireda | 9.5% | 5.1% | 22.4% |
|
a Rescue devices utilized: DL + bougie, 11 cases; LMA, 25 cases; FFB, 10 cases; VL, 26 cases; Cric, 3 cases.
Cric, cricothyroidotomy, DL, direct laryngoscopy; FFB, flexible fiber-optic bronchoscopy; LMA, supraglottic laryngeal mask airway (Teleflex); VL, video laryngoscopy |
|||
ETT tip abutment against the anterior/lateral subglottic wall (grade I, Figure 10), the vocal cords or arytenoids (grade II, Figure 11), or the pharyngeal wall (grade III) often resulted in the Murphy eye being the only portal for ventilation and oxygen delivery. Luminal narrowing due to accumulated biofilm and concretions led to use of a luminal cleansing device (CAM Rescue Cath, Omneotech) for debris removal to afford FFB management (n=3; Case 5, with Figures 12-14).
Reintubation of the grade III extubation was endangered by excessive secretions, periglottic edema, a partial or an impassable ETT tip due to impingement against airway structures, biofilm, and a confined hypopharyngeal space, singly or in combination (Table 5, Figures 15 [p. 46] and 16 [p. 47]). Bronchoscopy required an adjunct airway device for rescue, typically a video laryngoscope, due to circumstances related to an obstructed or impinged ETT tip complicated by secretions that interfered with visualization.
As stated above, FFB and a video laryngoscope were the most commonly used devices, and each had similar complication rates (Table 6). However, each complemented the other and served as a valuable and lifesaving rescue adjunct for the other. Bronchoscopy, overall, fared very well in all three grades of extubation encounters. Laryngoscopy, particularly VL, was beleaguered by a persistent management concern, noted during attempts to advance the existing ETT in a grade III extubated trachea. Two factors contributed to early difficulty following the introduction of VL into clinical practice: 1) the acute VL blade angle, and 2) the ETT’s softened consistency (thermolability). These two factors led to uncoordinated and unpredictable advancement of the existing “softened” ETT during grade III reintubation. The corrective action taken was to remove the old ETT and pass a new, styleted ETT under VL guidance. Appreciation and correction of this factor markedly reduced VL-related difficulties.
| Table 6. Complications by Management Method/Device | |||||
| Complication | DL (n=69) | VL (n=170) | AEC (n=18) | VL + AEC (n=15) | FFB (n=323) |
|---|---|---|---|---|---|
| Hypoxemia (<90%) | 17.6% | 7.2% | 11.1% | — | 7.5% |
| Hypoxemia (<80%) | 20.6% | 4.8% | 50.1% | — | 4.8% |
| Esophageal intubation | 32.4% | 2.4% | 50.0% | — | 1.0% |
| Bradycardia | 20.6% | 2.4% | 44.4% | — | 3.8% |
| Cardiopulmonary arrest | 13.2% | — | 22.2% | — | — |
| 3+ or more attempts | 33.3% | 1.3% | 27.8% | — | 6.5% |
| Rescue device required | 43.5% | 2.9% | 61.1% | VL alone (4) | 11.4% |
| AEC, airway exchange catheter; DL, direct laryngoscopy; FFB, flexible fiber-optic bronchoscopy; VL, video laryngoscopy | |||||
Use of the AEC alone, without laryngoscopic assistance, led to a significant number of life-threatening complications. Several factors contributed to the majority of these complications. First and most importantly, there were several instances in which the AEC was blindly advanced via the indwelling ETT to either assist with its exchange (assuming a cuff perforation) or afford advancement of a proximally migrated ETT. These two clinical situations exemplify why it is imperative to perform an airway assessment prior to any corrective maneuvers. The database, while certainly not a randomized controlled evaluation of airway interventions, offers many examples that clearly identify maneuvers to clarify the ETT location/position that are absolutely imperative to the safety of the patient and the smooth execution of the airway intervention (Case 6 [p. 48]).
Another concern when employing a straight-tipped AEC was identified by the airway team: Despite the luxury of adequate periglottic visualization with VL, there was difficulty navigating the AEC tip through the glottic opening. This hampered reintubation efforts (grade III). Of note, a frequent clinical observation was when the ETT tip was found to be lined up directly above the glottic opening. Passing of the more rigid AEC down the softened ETT led to unpredictable ETT repositioning within the hypopharynx, thus moving the tip away from its previously “optimal” position. Otherwise, a new styleted ETT or FFB should be used to resecure the airway. An angled-tip catheter (i.e., a bougie with coudé tip) may be a better choice—and only if able to visualize the glottic structures—but was not evaluated. The corrective action taken in the grade III extubations was to abandon passing an AEC and encourage an FFB- or VL-based airway evaluation to discern the etiology of the cuff leak. Again, grade I and II partial extubations can be managed successfully with the AEC.
Ready access to other airway management adjuncts is central to providing rescue airway management that frequently accompanies critically ill patients outside the OR environment.8-10Primary and secondary supraglottic airway devices played an important backup role for management difficulties.8-10 Moreover, there was one case in each of the three grades of partial or complete tracheal extubations that required a rescue surgical airway (cricothyrotomy). Airway and hemodynamic complications were nearly nonexistent in the remaining patients who were evaluated for a cuff leak that proved not to be associated with partial or complete tracheal extubation.
Miscellaneous Findings
Three clinically relevant findings commonly observed with partial or complete extubations deserve to be discussed:
- Checking the degree of pilot balloon inflation at the time of cuff leak evaluation will prove informative.6 If the pilot balloon is intact and remains inflated, it essentially rules out a cuff perforation as the etiology of the leak. However, there were eight cases in which the cuff leak was based on simultaneous ETT displacement (grades I-III) and cuff perforation (n=4) or a dysfunctional pilot balloon (n=4). Again, an airway assessment with FFB and/or VL is essential.
- The vast majority of measured cuff pressures in the displaced ETT were in the normal range of 20 to 30 cm H2O pressure. The cuff inflation volumes, however, were variable with the extubation grade. Grade I displacement typically required the removal of 10 to 20 mL of air to afford cuff collapse, compared with 35 to 125 mL in grades II and III, due to the expansive supraglottic–hypopharyngeal space.6 Incremental additions of air to the cuff to combat a leak likely potentiate a grade I ETT to migrate to grade II/III. Moreover, incremental air injection likely promoted slow cuff stretching to alter its compliance.
- As previously stated, the depth marking of the ETT, based on the dentition/gingival line, had little correlation to the ETT tip depth in those patients with partial/complete tracheal extubations. Of note, the ETT tip depth in all three grade categories was uncommonly shallow, with the vast majority showing ETT depth markings greater than 22 cm at the dentition/gingival line. Thus, the depth markings at the dentition/gingival line appear to be a misleading finding and probably should not be used solely as a criterion to estimate ETT depth.
Another interesting finding was an uncommon but untimely mishap taking place during airway assessment with a laryngoscope. There were several cases of perforation and rapid deflation of the herniated cuff located in the hypopharyngeal space (grade III) by the placement of a laryngoscope during the preprocedural airway assessment. Three cases of cuff perforation were documented (DL, 2; VL, 1).
Discussion
The differential diagnosis of a cuff leak is varied and requires vigilance and careful investigation. Assuming a particular etiology (e.g., cuff microperforation) may prompt the airway team to advance an AEC alone without laryngoscopic assistance, without the benefit of a preexchange airway assessment, through an unrecognized grade III ETT dislocation (Case 6 [p. 48]). Meticulous attention to distinguish the many causes of such leaks is essential (Table 1).1Recently, El-Orbany and Salem elegantly reviewed ETT cuff leaks and their associated causes, consequences, and management.1 Cephalad migration of the ETT above the glottis may be the most frequent cause of cuff leaks in the ICU population.1
The preprocedural screening process uncovered a substantial number of patients with cuff leaks that the requesting ICU staff interpreted as a dysfunctional cuff (e.g., a perforation or faulty pilot balloon–valve assembly) rather than as cephalad migration of the ETT.7 This review lends support to performing a preprocedural laryngoscopy or bronchoscopy screening as a valuable component in the evaluation of a cuff leak.7
Despite the use of advanced airway management equipment and an experienced airway team, airway management of the critically ill population is challenging.11-16 Not surprisingly, managing cephalad migration of the ETT was clearly perplexing at times, unpredictable, and potentially life-threatening. Grade I ETT migration, in most cases, was handled deftly and efficiently with minimal consequence. Conversely, grade II and III migrations were more challenging, leading to life-threatening complications (Figure 17). Maintaining competent FFB skills and immediate access to such equipment augments its value in the ICU setting. Access to the larger diameter (>5 mm) FFB affords invaluable suction capabilities, maneuverability, and improved visualization.
Likewise, VL provided excellent results for grade I and II partial extubations and as a rescue alternative for FFB failures.7,17-20 Conversely, in the early stages following the acquisition and deployment of VL, VL-assisted reintubations for grade III cases were fraught with difficulties that led to significant airway and hemodynamic consequences.21 Forward advancement of the thermolabile, softened, nonstyleted (existing) ETT toward the glottic opening combined with the extreme 60- to 70-degree blade camera angle of the video laryngoscope may lead to erratic and poorly coordinated ETT delivery.
Once appreciated, this now ill-advised practice of advancing a nonstyleted and softened ETT in a grade III extubation situation should be handled with the advancement of a new, styleted ETT. Likewise, a cautious approach for grade II displacement is warranted, as three cases of esophageal intubation were documented when the advancement of a nonstyleted (existing) ETT, despite the ETT tip being located at the glottis, was deflected into the esophagus.
An additional caveat gleamed from this data review involves the combination of VL and the AEC in the management of grade III extubations. Despite the overall favorable use of VL with critically ill patients with a difficult airway,7,17-20 serious concern should be raised when using a video laryngoscope plus AEC in managing complete extubation of grade III cases. There was noticeable difficulty maneuvering the straight-tip AEC into the trachea due to the extreme 60- to 70-degree video laryngoscope blade angle and the confined hypopharyngeal work space, coupled with secretions, airway edema, and the lack of a bent tip (coudé) that hindered directional guiding capabilities. Further, the softened ETT tip–cuff complex in the hypopharynx may undergo unpredictable repositioning when the semirigid AEC is advanced through the ETT lumen. This factor contributed further to difficult reintubation.
Additionally, two important clinical points noted in this data review are worthy of mention, as they pose valuable diagnostic clues for HCPs: First, the depth markings on the ETT have little correlation to the actual depth of the ETT cuff and tip in partial/complete extubation. Therefore, assuming a depth of greater than 24 to 26 cm infers the ETT must be in a good position could be grossly erroneous. Second, an intact pilot balloon, present in 98% of the cuff leak cases, presents a potentially simple and accurate finding that may serve as a helpful clinical sign. This clinical finding offers an intriguing diagnostic clue to assist in differentiating a true cuff leak—cuff perforation or pilot balloon malfunction (incompetent pilot balloon, e.g., deflated)—compared with a dislocated ETT tip (intact pilot balloon, e.g., inflated). The authors have embraced this clinical finding as a component in the evaluation of a cuff leak but not as a sole determinant of unrecognized tracheal extubation.
Based on this data review, cephalad migration of the ETT in the ICU patient appears to be a relatively common etiology for a cuff leak, although an accurate denominator is not available to determine its true incidence.1 Partial (grades I and II) and complete (grade III) extubation of the trachea are high-risk airway problems that present significant safety concerns if left undetected. Moreover, corrective measures may expose the patient to life-threatening consequences. This airway quandary has not received its warranted attention regarding complications, patient outcomes, potential prevention strategies or continuous diagnostic monitoring (reflective Doppler technology).
Bronchoscopy is an accomplished diagnostic and therapeutic choice when performed by skilled operators who have backup strategies and rescue equipment alternatives. On the other hand, VL is an excellent option for both diagnostic visualization and reintubation of the trachea.21However, a few caveats are warranted for its use, especially in grade II and III extubation cases because of some confounding factors: ETT advancement may be hampered by its thermolabile and thus softened consistency, as well as its serpentine shape or arched positioning within the pharynx. Therefore, reintubation with a new styleted ETT rather than attempting to advance the existing, softened ETT may be preferred in grade II and III extubations. Moreover, despite VL visual confirmation of a grade III extubation, navigating the AEC’s straight tip from the ETT lumen placed in the pharynx may be difficult and lead to avoidable complications, so its use for this intervention should be discouraged. Even if the airway team finds the ETT tip is positioned in direct alignment with the glottic opening, advancement of the more rigid AEC will likely alter the softened ETT position and lead to difficult advancement. The passing of an airway catheter or a bougie with a coudé tip may be more successful, but was not evaluated in this review.
To summarize:
- One’s ability to gain visual access to the periglottic anatomy is a great leap forward toward potentially improving the safety margin of highly complex and challenging airway interventions.
- Education of all ICU staff and care providers is an essential element for early diagnosis.
- A preprocedural airway evaluation is warranted.7
- Evaluation of the pilot balloon cuff may render useful diagnostic information regarding the origin of the cuff leak.
- Despite a seemingly adequate depth at the dentition line, the ETT should be evaluated for potential displacement.
References
-
- El-Orbany M, Salem MR. Endotracheal tube cuff leaks: causes, consequences, and management. Anesth Analg. 2013;117(2):428-434.
- Rashkin MC, Davis T. Acute complications of endotracheal intubation. Relationship to reintubation, route, urgency, and duration. Chest. 1986;89(2):165-167.
- Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med. 1981;70(1):65-76.
- Zwillich CW, Pierson DJ, Creagh CE, et al. Complications of assisted ventilation. A prospective study of 354 consecutive episodes. Am J Med. 1974;57(2):161-170.
- Kearl RA, Hooper RG. Massive airway leaks: an analysis of the role of endotracheal tubes. Crit Care Med. 1993;21(4):518-521.
- Sultan P, Carvalho B, Rose BO, et al. Endotracheal tube cuff pressure monitoring: a review of the evidence. J Perioper Pract. 2011;21(11):379-386.
-
- Mort TC, Braffett BH. Conventional versus video laryngoscopy for tracheal tube exchange: glottic visualization, success rates, complications, and rescue alternatives in the high-risk difficult airway patient. Anesth Analg. 2015;121(2):440-448.
- Popat M, Mitchell V, Dravid R, et al. Difficult Airway Society guidelines for the management of tracheal extubation. Anaesthesia. 2012;67(3):318-340.
- Law JA, Broemling N, Cooper RM, et al. The difficult airway with recommendations for management—part 2—the anticipated difficult airway. Can J Anaesth. 2013;60(11):1119-1138.
- Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2013;118(2):251-270.
- Griesdale DE, Bosma TL, Kurth T, et al. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008;34(10):1835-1842.
- Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults. A prospective investigation of 297 tracheal intubations. Anesthesiology. 1995;82(2):367-376.
- Le Tacon S, Wolter P, Rusterholtz T, et al. Complications of difficult tracheal intubations in a critical care unit [in French]. Ann Fr Anesth Reanim. 2000;19(10):719-724.
-
- Martin LD, Mhyre JM, Shanks AM, et al. 3,423 emergency tracheal intubations at a university hospital: airway outcomes and complications. Anesthesiology. 2011;114(1):42-48.
- Mort TC. Complications of emergency tracheal intubation: hemodynamic alterations—part I. J Intensive Care Med. 2007;22(3):157-165.
- Mort TC. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99(2):607-613.
- McLean S, Lanam CR, Benedict W, et al. Airway exchange failure and complications with the use of the Cook Airway Exchange Catheter®: a single center cohort study of 1177 patients. Anesth Analg. 2013;117(6):1325-1327.
- Benumof JL. Airway exchange catheters: simple concept, potentially great danger. Anesthesiology. 1999;91(2):342-344.
- Hagberg CA, Artime CA, Aziz MF. Airway management in intensive care medicine. In: Hagberg and Benumof’s Airway Management. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Mort TC. Tracheal tube exchange: feasibility of continuous glottic viewing with advanced laryngoscopy assistance. Anesth Analg. 2009;108(4):1228-1231.

- Aziz MF, Healy D, Kheterpal S, et al. Routine clinical practice effectiveness of the Glidescope in difficult airway management: an analysis of 2,004 Glidescope intubations, complications and failures from two institutions. Anesthesiology. 2011;114(1):34-41.
Case 1.
Called to “change the ETT” in a 62-year-old man with known metastatic esophageal cancer with local/regional spread. The patient was intubated two days prior for pneumonia with FFB due to 2-cm trismus, no cervical range of motion, and front of neck anatomy that was woody, thickened and immobile, with induration spreading down to the clavicles.
Having been asked to exchange the ETT, the airway team planned on passing an AEC via the indwelling ETT and performing the exchange without laryngoscopic assistance. As they prepared, one team member, who recently attended an airway seminar, noted the cuff appeared properly inflated and suggested that the next step prior to exchanging the ETT was to verify the ETT position. There was disagreement among the team members, but persistence paid off.
As VL would not yield useful information due to the degree of trismus, a bronchoscope was passed via the ETT. The distal ETT tip was abutted against the lateral pharyngeal wall and ventilation was maintained via the Murphy eye (grade III extubation). After multiple attempts to reposition the ETT, it was eventually drawn proximal enough to allow advancement of the bronchoscope into the trachea (significant periglottic distortion and edema were noted).
Case 2.
The airway team was called to evaluate an 85-year-old man with severe chronic obstructive pulmonary disease, interstitial lung disease and pneumonia with a new-onset cuff leak. Intubated for 12 days, he remained dependent on mechanical support due to malnutrition and his respiratory limitations.

The 8.0-mm ETT was located at a depth of 21 cm. A chest radiograph (12 hours before) had shown the ETT was 5 cm above the carina and that there was a tortuous and calcified tracheal outline. The pilot balloon was intact. Bronchoscopy confirmed the presence of an orogastric tube within the trachea. The team removed the orogastric tube and the leak disappeared.
However, as the team was cleaning up after the procedure, a leak was noted again. Once again, the bronchoscope was passed and the ETT tip was noted at 5 cm above the carina. The ETT was advanced to 2 cm above the carina. The leak was less, but that was with the pilot balloon inflated to an intra-cuff pressure of 70 to 75 cm H2O. Longer-term plans for a tracheostomy were to be presented to the family the following day.
Following discussion with the ICU team, the airway team exchanged the 8.0 mm for a 9.0-mm ETT over a 19 Fr AEC with VL assistance. The cuff leak resolved while maintaining normal intra-cuff pressures.
Case 3.
The airway team was called to evaluate a new-onset cuff leak in an 82-year-old woman (5 feet tall, 135 pounds) with congestive heart failure and respiratory failure. She had been intubated for two days and mechanically ventilated with a 7.5-mm ETT at a depth of 23 cm and was now recording a 40% volume loss with each breath.
The previous day’s chest radiograph showed the ETT tip 3 cm above the carina. The pilot balloon appeared intact. An audible leak was heard with the intra-cuff pressure at 25 cm H2O. Additional air placed via the pilot balloon did little to abate the leak.
The airway team reviewed the intubation record (“easy intubation”). They then performed a VL-based airway assessment and found the ETT was properly positioned through the glottis; the periglottic anatomy was without appreciable edema or alteration.
Bronchoscopy revealed the ETT tip just entering the right main stem bronchus. The ETT was withdrawn to 2.5 cm above the carina and the leak disappeared (cuff pressure at 20-22 cm H2O).
Case 4.
The anesthesia airway team was called at 2 a.m. for a cuff leak in a 68-year-old man, status post-esophagogastrectomy two years previously, who had undergone radiation therapy and was now intubated for aspiration pneumonia. Although he has been improving and weaning, he remained on antibiotics. The patient just spiked a temperature to 102.8∞ F. A chest radiograph 10 minutes prior to the team’s arrival showed a right lower lobe infiltrate and the ETT tip 4 cm above the carina.
-
- Differential diagnosis: low cuff pressure, cuff perforation, ETT displacement (unlikely), damaged ETT, ETT–tracheal wall incongruity. No new probes or nasogastric tube placed recently.
- Airway exam: ETT at 25 cm, pilot balloon inflated/intact, bubbling with mechanical breath.
- Planned intervention: VL and bronchoscopy.
- Intervention: VL showed minimal secretions, ETT positioned within the glottis, and bubbles from the posterior glottic area. Bronchoscopy showed the ETT tip 3 cm above the carina, bubbling with each mechanical breath. The ETT was withdrawn proximal to evaluate the trachea where a posterior tracheal wall erosion/perforation was noted (likely communicating with reconstructed esophageal remnant/mediastinum).
- The thoracic surgical team was alerted. Due to the recent temperature spike and rising white blood cell count, the patient was taken emergently to the OR for diagnostic evaluation of the tracheoesophageal fistula and to control any mediastinal soilage.
Case 5.
The anesthesia airway team was called stat to the ICU for a cuff leak in a 47-year-old male trauma victim: one day status post–cervical spinal fusion, with a body mass index of 43 kg/m2. He was a difficult intubation on presentation to the emergency department, ++facial edema, “no neck,” and a hard cervical collar was present prior to corrective surgery. He was weaned down to continuous positive airway pressure support but had obvious bubbling from the mouth and SpO2at 90% to 92%, sitting upright in bed.
- The airway team considered the differential diagnosis: low cuff pressure, cuff perforation, ETT displacement, damaged ETT.
- Action: called for surgical backup; difficult airway cart ready at bedside.
- Airway exam: ETT at 26 cm, pilot balloon inflated (ETT displacement most likely).
- Planned intervention: both diagnostic and therapeutic bronchoscopy due to position and minimizing induction medications.
Patient then phonated in a whispery, high-pitched voice, “Is anything wrong?” Differential diagnosis narrowed down to grade III tracheal extubation. Intervention: bronchoscopy.
Case 6.
The anesthesia airway team was called for a “tube change because of a cuff leak” in a tall, slender, 44-year-old woman with severe necrotizing pancreatitis, septic shock, and acute respiratory distress syndrome (fraction of inspired oxygen, 60%; positive end-expiratory pressure, 16 cm H2O; sedated with propofol, lorazepam and hydromorphone infusions). The patient’s initial intubation was documented as a full view with a Macintosh 3 conventional blade.
Based on the request, a large 19 Fr AEC was advanced via the indwelling ETT. The old ETT was exchanged for a new 8.0-mm ETT with ease. No end-tidal carbon dioxide was detected and rapid desaturation ensued, leading to hypoxic bradycardia cardiac arrest.
Multiple attempts at intubation led to repeated esophageal intubations. A supraglottic airway device (LMA; Teleflex) was placed, oxygenation was restored, and cardiopulmonary resuscitation was halted. Tracheal intubation was successful with VL assistance.
In retrospect and following debriefing, several members of the team were aware that the pilot balloon had been intact, but this fact was ignored. Moreover, a pre-exchange airway assessment was not performed, and laryngoscopy was not used to assist with the exchange over the airway catheter. The team erroneously assumed that the patient’s tall and slender habitus and a previously documented “easy intubation” still applied (despite five days of mechanical ventilation and a positive fluid balance of 14 L since her ICU admission).




Leave a Reply
You must be logged in to post a comment.