Authors: Qisheng Ou, Ph.D. et al
Anesthesia Patient Safety Foundation
Disclaimer: We aim to present letters from our readership that may generate further discussion on managing patients with COVID-19. Given the novelty of COVID-19, best-available clinical evidence is limited and supported from anecdotal reports from China, South Korea, Italy and other sites, and from studies of previous epidemics like SARS and MERS. The opinions expressed are those of the authors and not the APSF. These materials are presented for informational and educational purposes only and do not establish a standard of care or constitute medical or legal advice. The APSF does not support or endorse any specific idea, product, equipment, or trademarked technique. We strongly promote consistency with your governing bodies and organizations such as the CDC, WHO, ASA, AANA, and AAAA. Readers are reminded to consult with their institutions and medical/legal advisors regarding any of the views and opinions expressed by the authors.
There is a serious shortage of respirators and masks that are essential for the protection of frontline healthcare workers and for the mitigation of community transmission during this Covid-19 pandemic1. Reuse of disposable filtering facepiece respirators and surgical masks after decontamination has become a necessary strategy2,3. We provide here scientific data to support the use of three decontamination methods.
The ultraviolet germicidal irradiation (UVGI) method4 was implemented with two UV systems (Clorox Optimum-UV Enlight® System, 216 mJ/cm2) located 1 meter (3.3 feet) away from the front and back of the respirators/masks hanging in the middle of a small decontamination room. It generated UV-C light and irradiated the masks for 5 minutes. For the oven heating method, 77oC (170oF) was chosen because it is the lowest temperature setting for most household ovens, and Covid-19 virus is deactivated at 70oC5. At the target temperature, the respirators/masks were placed on a stack of coffee filters inside the oven without touching any metal surfaces to prevent thermal damage and heated for 30 minutes. For the steam heat treatment method, the respirators/masks were placed on the rack of a steamer with boiling water for 30 minutes. This treatment should not be done in a microwave oven because the metal nose clip may damage both the respirators/masks and the microwave oven.
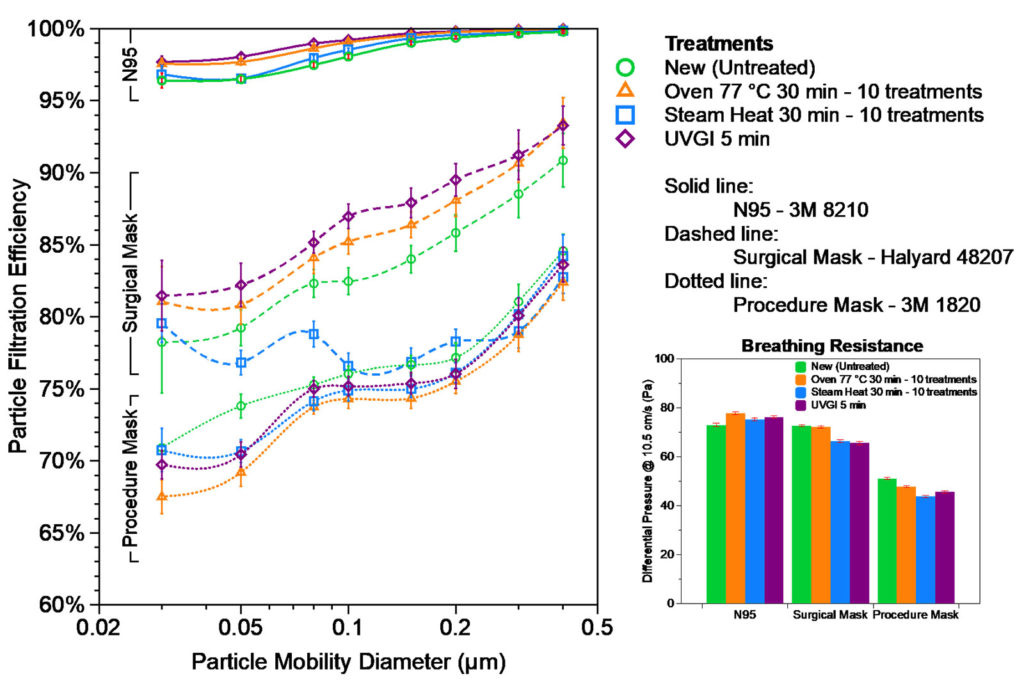
Filtration efficiency and breathing resistance of 3M 8210 N95 respirator (St. Paul, MN.), 3M 1820 procedure mask, and Halyard 48207 surgical mask (Alpharetta, GA) were measured before and after the decontamination treatment. Although Covid-19 virus is ~ 0.1 µm in size6, the exhalation droplets can be several micrometers or larger but shrink while traveling in air due to water evaporation. Efficiency of filter media is a strong function of contaminant size. We report here fractional efficiency for different sizes from 0.03 to 0.4 µm, which represents the most penetrating particle size range, so that it can be compared to the size of Covid-19 virus or other pathogens of interest. As shown in Figure 1, N95 respirator has >95% efficiency over the entire size range, with the least efficiency of 96% at 0.05-0.08 µm, and is >98% efficient at Covid-19 virus size of ~ 0.1 µm. Surgical mask and procedure mask have lower efficiency with ~85% and ~80% at 0.1 µm, respectively. All three decontamination treatments did not cause visible deformation or degradation of the material nor did they degrade filtration efficiency or breathability after as many as 10 treatments. The only exception is that the steam heat treatment caused slight efficiency drop (<5% on the average) on surgical masks after 10 treatment cycles, suggesting that oven heating is a better option for repetitive reuse. The three decontamination methods were tested safe in retaining filtration of most household fabric materials (data not shown) that could also be used for home-made masks. Our data indicates there is no systematic change of efficiency or resistance on N95s caused by the treatment. The slight increase in resistance of treated N95s is from sample variation, rather than the treatment itself. The test method is destructive, so we limited our sample counts to save precious N95s and masks.
Figure 1. Fractional Particle Filtration Efficiency and Breathing Resistance (Differential Pressure) of the decontamination treated samples of 3M 8210 N95, Halyard 48207 surgical mask, and 3M 1820 procedure mask, compared with the new untreated samples.
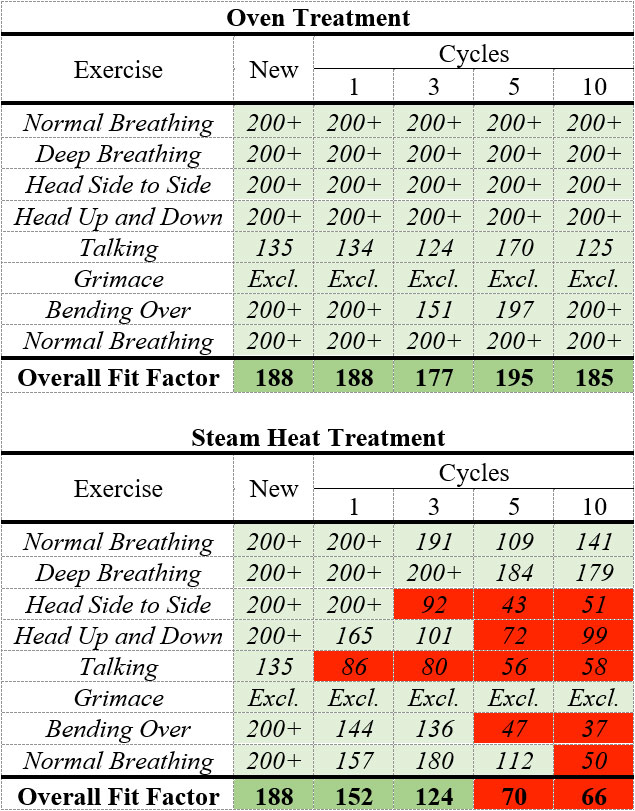
The quantitative fit tests were performed using a TSI PortaCount® Pro+ 8038 by a specific researcher in this study. The fit factor, defined as the ratio of ambient particle concentration to the particle concentration inside the respirator, should be equal to or above 100 to pass the test. The quantitative fit testing was first performed with a new 3M 8210 N95 respirator and then performed after 1, 3, 5, and 10 cycles of 77oC oven treatment with the same respirator. A second 3M 8210 N95 respirator was fit tested after 1, 3, 5, and 10 cycles of steam heat treatment. As shown in Table 1, oven treatment deemed safe for the integrity and the fit of the respirator, while the steam heat treatment may affect the respirator fit. All the fit tests were performed with the same person. Different fit factors should be expected if the tests were performed on a different wearer, even if with the same respirator. During the fit testing, the person who conducted the tests did not feel any difference in terms of breathability between untreated and treated N95s.
Table 1. Quantitative fit testing results of the new N95 respirator and after oven and steam heat treatment cycles.
Conclusion
We tested three methods (UVGI, Oven, and Steam Heat) for decontamination and found that they did not degrade the filtration efficiency and fit factor. Based on our present findings, the reuse respirators and masks are not only highly efficient but can be used repeatedly for up to 10 times. Moreover, the methods are readily available not only in the hospital setting but also in most home environments. This study only tested unused respirator/mask performance after multiple decontamination treatments. Worn respirator/mask may have deterioration in integrity and efficiency, which cannot be recovered from decontamination. We do not recommend reusing N95s or masks that are visibly contaminated or with visible deterioration on any part of the materials.
References
- Livingston E, Desai A, Berkwits M. Sourcing Personal Protective Equipment During the COVID-19 Pandemic. JAMA. Published online March 28, 2020. doi:10.1001/jama.2020.5317
- Decontamination and Reuse of Filtering Facepiece Respirators. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html.
- Coronavirus (COVID-19) Update: Reusing Face Masks and N95 Respirators: JAMA. Published online April 8, 2020. URL: https://edhub.ama-assn.org/jn-learning/audio-player/18433414
- Mills D, Harnish DA, Lawrence C, Sandoval-Powers M, Heimbuch BK. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. American Journal of Infection Control. 2018;46(7):e49-e55.
- Chin AWH, Chu JTS, Perera MRA, et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. Published online April 2, 2020. doi:10.1016/S2666-5247(20)30003-3
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727-733.


Leave a Reply
You must be logged in to post a comment.