Introduction
Cold (nonsurgical) laser therapy is a form of photobiomodulation (PBM) therapy, which can be used for the treatment of both acute and chronic pain. Compared with alternative therapies like acupuncture (ca. 8000-3500 B.C.), chiropractic (1895), and even physical medicine and rehabilitation (1947), cold laser therapy is a relatively recent development in medicine. The first cold laser was FDA-approved for treating pain in 2001, and low-level laser therapy (LLLT) has only been widely used in clinical practice in the United States since 2002. High-intensity laser therapy (HILT) is a more recent development, with the first publications appearing in 2009.1 LLLT using Class II and III lasers (producing <500 mWatts [mW] or <0.5 W of power) is a noninvasive, painless and easily administered therapy for a wide variety of superficial clinical conditions (Tables 1-3).2 It has been promoted as an effective way to produce analgesia and accelerate healing when used as an adjuvant to both pharmacologic analgesics and physicotherapy.2-4
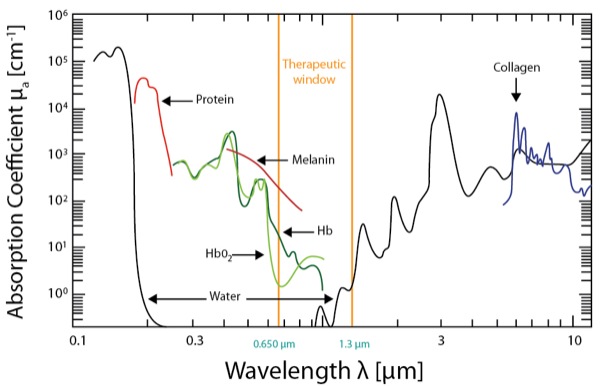
On the other hand, HILT devices are more powerful Class IV lasers producing >40 W of power at longer wavelengths (e.g., Phoenix Thera-lase [1,275 nm] and CureWave [1,280 nm]), thereby allowing deeper tissue penetration (Table 1). These devices have been introduced as noninvasive treatment modalities for both acute and chronic pain requiring deeper tissue penetration (Table 1).8 HILT therapy has been used to treat degenerative joint conditions and a wide variety of musculoskeletal disorders.8-11 HILT lasers work by administering a focused laser beam with peak power in the range of 20 to 75 W (which represents the maximum optical power of a given pulse) at longer wavelengths of light (>800 nm).12-13 The newest HILT device is manufactured by CureWave Lasers LLC (Dallas), and provides a maximum 44 W of power at a wavelength of 1,280 nm. The main advantage of the CureWave HILT device is that it can come in closer contact with the skin without overheating or burning the skin. The wavelength is important because higher wavelengths have reduced absorption of the laser beam by melanin, oxyhemoglobin and hemoglobin (Figure 1), thereby allowing deeper penetration into the soft tissue and muscle. With more powerful lasers, which also function at longer wavelengths, HILT devices can effectively stimulate larger tissue areas while also penetrating more deeply into the soft tissue (Figure 1).14Although there are several different commercially-available HILT devices (Figures 2-7), not all of these devices have been approved for clinical use by the FDA.
| Table 1. Characteristics of Cold Laser Devices Used for Providing Low-Level Laser Therapy and High-Intensity Laser Therapy | ||
| Feature | Low-Level Laser Therapy | High-Intensity Laser Therapy |
|---|---|---|
| Laser class | I, Im, II, III | IV |
| Wavelength | 600–980 nm | 660–1,280 nm |
| Power | <1 W | 1-75 W |
| Penetration abilities | Low (<2 cm) | Deep (5-15 cm) |
| Temperature changes | <1.0°C | Low thermal accumulation |
| Table 2. Commercially Available Cold Laser Systems | ||
| Brand Name | Wattage | Wavelength |
|---|---|---|
| Apollo | 0.5-20 W | 810 nm |
| Aspen Summit | 1-60 W | 810-980 nm |
| Avant | 0.66-1.4 W | 808 nm |
| BioLase Epic | 10 W | 940 nm |
| BioLight Aura PTL | 5 mW | 635 nm |
| BTL 6000 High Intensity Laser | 10 W | 1,064 nm |
| CureWave HILT | 1-44 W | 1,280 nm |
| Cutting Edge | 1.1-3.3 W | 810 nm |
| Erchonia XLR8 laser | 7.5-20 mW laser diodes | 635 nm |
| Ga–Al–As laser | 50 mW | 809 nm |
| Infrared laser IR810/400 probe | 400 mW | 808 nm |
| Irradia Mid-Laser | 120 mW | 660 nm |
| 20 W | 904 nm | |
| K-Laser | 6-20 W | 660-980 nm |
| Light Force/LiteCure | 10-25 W | 810/980 nm |
| Lumix Series 2-3 | 45-250 W | 810-910 nm |
| Multiradiance LaserStim | 7.5 mW | 660 nm |
| 25 W | 905 nm | |
| Nd:YAG laser | 0.25 W | 1,064 nm |
| Nexus | 10 W | 810-980 nm |
| Phoenix Thera-Lase | 37-75 W | 1,275 nm |
| Pilot Diode Laser | 9 W | 810-980 nm |
| R650/50 probe | 50 mW | 658 nm |
| TerraQuant | 15-50 W | 660-905 nm |
| THOR Laser | 150 mW | 660 nm |
| 2 W | 810 nm | |
| mW, milliwatt; nm, nanometer; W, watt | ||
| Table 3. Clinical Applications of Low-Level Laser Therapy and High-Intensity Laser Therapy for Acute and Chronic Pain | |
| Low-Level Laser Therapy | High-Intensity Laser Therapy |
|---|---|
|
Note: Several of the applications listed for LLLT have also been successfully treated with HILT. Although some of these applications have not been formally studied using a HILT device, there is no reason to expect that these conditions would not respond as well or even better compared with LLLT.
|

Proposed Mechanisms of PBM Therapy
PBM therapy has been studied for over 40 years. The more recently introduced form of PBM, namely cold laser therapy, has been demonstrated to produce an anti-inflammatory effect which promotes tissue healing and reduces pain. Animal studies suggest that cold lasers also promote fibroblast proliferation and the synthesis of Types I and III procollagen mRNA, which hastens bone healing and facilitates wound revascularization.15
The proposed mechanism of action of laser therapy relates to the ability of damaged cells to absorb the emitted photons of light and transform the energy into ATP (Table 4). Laser stimulation enhances the production of ATP by forming singlet oxygen and reactive oxygen species.15 The light-absorbing components of the cell are termed chromophores (i.e., photo-acceptors or photon receptors) and are contained within the mitochondria and cellular membrane. Cell components (e.g., cytochrome c, porphyrins and flavins) also have light-absorbing capacity.
The proposed mechanism of LLLT is related to electronic excitation of chromophores via cytochrome c oxidase (unit IV in the mitochondrial respiratory chain), which modulates a redox status of the molecule to enhance cellular functional activity. The chromophores contain both heme and copper centers that absorb light in the near-infrared region. The light-emitted photons dissociate inhibitory nitric oxide from the cytochrome c oxidase enzyme leading to an increase in electron transport and the mitochondrial membrane potential, thereby enhancing ATP production. The energy density ( joules[J]/cm2) correlates with the efficiency of laser radiation. The energy density is also responsible for regulating or “speeding up” the transport of electrons in the mitochondrial respiratory chain.16
The World Association of Laser Therapy has established that target tissues need an energy density of 5 to 7 J/cm2 to elicit a biological cellular response.17 Several studies using cold laser therapy for musculoskeletal pain have shown that better therapeutic effects of laser therapy are achieved with higher energy density (i.e., HILT), as well as by administering additional laser treatment sessions.18,19 Huang et al20 also demonstrated that the beneficial outcomes of cold laser therapy were related to the amount of energy (J) administered to the injured tissue/nerves.
Additionally, HILT may have a direct stimulatory effect on nerve structures, which could increase the speed of recovery from conduction block or inhibition of A-delta and C-fiber transmission.21Chow and Armati22 reported that animal studies using noxious stimuli indicate that HILT devices produce nociceptor-specific inhibition of nerve conduction, leading to inhibited transduction of pain signals from the periphery to the central nervous system. In addition, there is increasing evidence that laser therapy can disrupt neuronal physiology, affecting axonal flow and cytoskeleton organization. These laser-induced changes are completely reversible with no side effects or residual nerve damage.
In summary, laser-induced analgesia appears to be based on the capacity of infrared light to modulate various metabolic processes by converting the laser light energy through biochemical and photo-physical processes to transform the laser light into energy, which is used to promote cellular healing.21
Techniques Used for Administering Laser Therapy
Multiple variables affect the clinical therapeutic effects of infrared laser treatments, including energy density, wavelength, laser delivery system, the number of treatment sessions and their duration.23 The lack of consistency with respect to controlling these important parameters contributes to the inconsistent results reported in the peer-reviewed literature. It has also been suggested that a therapeutic window exists in order to generate effective photo-stimulation in order to achieve the therapeutic benefits of PBM therapy.23 Other important issues that require further study include determining the effects of laser therapy on the activity of abnormal cancer cells when laser therapy is used for treating cancer-related pain, as well as the effects of laser therapy in patients with concussions and chronic neurodegenerative diseases.
The therapeutic laser dose depends primarily on three factors: power output, wavelength and time (i.e., duration of treatment sessions).17 These factors have also been reported to have the greatest influence in enhancing tissue healing and improving clinical outcomes.20,24-25Surprisingly, the optimal laser dose required to produce the desired photo-stimulating effect on specific body regions remains largely unknown. Achieving a therapeutic dose without under- or overstimulating the target tissues is one of the most challenging aspects in administering PBM therapy.25 If the amount of energy absorbed is insufficient to stimulate the absorbing tissues, no reactions or changes can occur in the targeted body tissues. Weak stimuli (i.e., underdosing) produces little or no effect on cellular function, moderate to strong laser stimuli positively enhance cellular function, while excessively strong stimuli (i.e., overdosing) can suppress or inhibit cellular function and further damage the tissue due to the adverse effects of overheating the tissue.20 Future research is needed to determine the optimal doses and frequency/duration of cold laser treatments for treating acute and chronic pain with both LLLT and HILT.
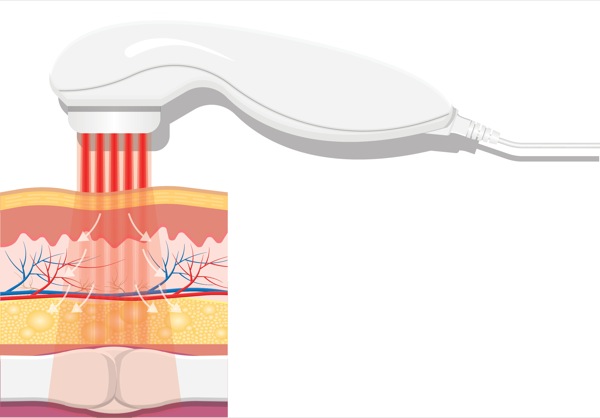
Wavelength is obviously an important parameter because it determines the ability of a laser beam to penetrate tissue (Figure 1 and Table 4).26 When light therapy is administered directly to the patient’s skin, some of the laser light effect is attenuated by superficial tissue layers due to absorption into skin pigments (e.g., melanin). Joensen et al27 revealed that the amount of penetrating light energy is 20% for a wavelength of 810 nm and 58% for a wavelength of 904 nm. Tuner and colleagues have analyzed the reasons for negative studies with LLLT.28-31 For example, the majority of light energy (50% to 90%) was absorbed by the skin and subcutaneous tissues and only 10% to 50% penetrates into the deeper tissue layers, depending on the wavelength.
Diodes with wavelengths ranging from 400 to 700 nm can transmit light energy only to the epidermal and subdermal tissue layers (<1 cm) and are therefore best suited for treating superficial wounds and skin disorders. These lasers can also lead to burning when they are in close contact with the skin surface. Laser diodes with wavelengths ranging from 820 to 904 nm can transmit light energy from 2 to 4 cm beyond the skin surface. Lasers with higher wavelengths (>1,000 nm) are best suited for treating deep soft tissue injuries (e.g., muscle, deep fascia, ligaments, joints).24,32-33
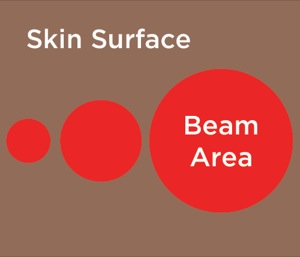
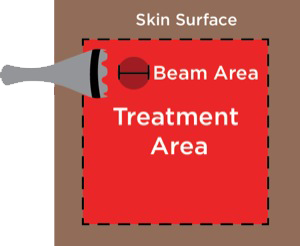
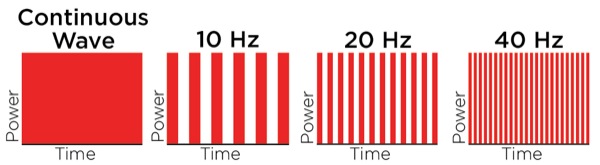
In contrast to LLLT, HILT is delivered either in short bursts (120-150 msec) or continuously at 60-sec intervals, with a brief duty cycle (0.1%), to prevent the laser from reaching the thermal tissue threshold while allowing time for adequate thermal relaxation (Table 4). This is important for preventing superficial tissue damage and avoids potentially harmful thermal accumulation leading to tissue damage from overheating of the soft tissue. Additionally, the pulsed delivery of light allows higher doses of photo-energy to reach deeper tissues, particularly at short pulsed and low repetition rates.34 For example, a 905-nm continuous wave infrared laser allows 2.5 cm penetration of a clinically effective dose of photo-energy, while a 905-nm super-pulsed infrared laser can achieve a depth of 10 cm for nanoseconds.34 However, with the use of higher wavelengths (>1250 nm) it is possible to administer longer pulse durations, up to and including a brief continuous wave lasting 60-80 sec to achieve deeper tissue penetration for a more sustained period of time, resulting in a greater clinical effect without producing tissue damage. High-power longer pulsed continuous emission provides broader coverage (i.e., wider treatment area) and a greater therapeutic photomechanical effect. The wider coverage provided with the newer more powerful HILT devices also reduces treatment times.
Cold laser therapy is administered using a simple “point and shoot” technique at the involved tissue area(s) and/or nerves. In addition to accurately identifying the painful area (as well as the nerve innervation to the symptomatic area [i.e., peripheral nerve roots]), other important variables to consider in administering laser therapy are the optimal power level (W) and wavelength (nm) of the laser beam itself to achieve adequate tissue penetration, treatment times, and number of initial and follow-up treatment sessions. The laser technician administering the treatments must be trained in laser safety procedures and have background training in anatomy, kinesiology and/or physical therapy. After identifying the painful area, the laser beam is pointed directly at the overlying skin area at varying distances depending on the power and wavelength of the laser device. The low-power lasers used for LLLT can be placed in direct contact or within 3 to 5 in of the skin surface. However, direct contact of the laser head with the skin can potentially lead to cross contamination between patients. Typically, these superficial LLLT treatments require 5 to 20 min.
However, the more powerful HILT devices require that the laser probe be held a distance of 10 to 12 in from the skin surface to avoid overheating the treated area. The designated body area(s) are treated with a series of interrupted continuous 60-sec treatments to cover the symptomatic area (the exact distance depends on the width of the laser beam). With the current HILT devices, the width of the laser beam varies from 2.5 up to 5 cm. Limiting the HILT treatment times to 60 to 80 sec at each point will avoid overstimulating the tissue, which leads to tissue fatigue and release of muscle enzymes.
Depending on the size of the painful area and/or number of symptomatic body areas, a HILT treatment session can last from 10 to 45 min. The patients may be asked to perform simple range of motion exercises immediately before, during and after the laser treatment session if the treatment involves a tendon or joint to optimize the treatment response. Typically, the analgesia following a single laser treatment session lasts 2 to 4 days, and with repeated treatments the analgesic effect can be prolonged 7 to 10 days or longer. Although some acute injuries resolve after only 1 to 2 treatment sessions, most chronic pain conditions require a series of 8 to 12 treatment sessions over 3 to 4 weeks, followed by maintenance treatment every 3 to 5 weeks for progressive conditions. Interestingly, we have found that the clinical pain-relieving effects of HILT in patients with chronic pain are similar to the effects achieved with acustimulation using needles at either acupoints or dermatomes.2 However, the magnitude of the acute response to HILT appears to be greater than acustimulation and the beneficial effect is longer lasting.
Laser beams target surgical incision sites for postoperative analgesia (and to stimulate faster wound healing), painful trigger points, acupoint sites associated with the painful symptoms, paraspinous region of the spinal column, peripheral nerves innervating areas of numbness and pain (e.g., neuropathic pain), and/or broad soft tissue coverage to reduce localized pain and inflammation, thereby stimulating tissue and peripheral nerve healing.35 From a safety standpoint, the most important consideration is to avoid direct exposure of the laser beam to the eye, which will cause retinal damage. Special protective goggles should be worn by both the patient and the operator during the treatment sessions. These goggles should block the wavelength of the cold laser system being used and can be purchased from laser safety manufacturers (e.g., Laservision USA). All laser technicians should be certified in laser safety procedures by completing an online laser training course (e.g., Thor Labs’ cold laser training program, found at www.thorlaser.com/?courses/?index-US.php).36
Evidence-Based Clinical Applications of Cold Laser Therapy
Osteoarthritis
Cold laser therapy has been proven to reduce pain and improve physical disability associated with osteoarthritis (OA) of the knee,4,9,37-39 even prolonging the time to needing knee replacement surgery40 and improving physical activity.9,37,38 Other OA studies in former professional athletes have demonstrated reduced pain and improved activity after only a few treatment sessions.11,41,42
Hemophilia-Induced Arthritis
Hemophilia-induced arthropathy due to recurrent joint bleeding leads to physical, psychological and socioeconomic problems in children. HILT reduced pain, increased functional capacity, and improved gait performance in children with hemophilic arthropathy after only a few treatment sessions.43,44
Chronic Low Back Pain
Low back pain is a major cause of medical expenses, absenteeism and disability, with an estimated prevalence in developed countries of 23%, and 48% in low and middle income countries.45,46 HILT has been shown to be highly effective in patients with musculoskeletal back pain,47 improving pain and functionality and reducing disability.14,48,49 However, some studies have found that LLLT fails to provide pain relief or improve functional capacity in patients with low back pain.50-52
Chronic Neck Pain
Neck pain is a common and costly condition for which pharmacologic management has limited evidence of efficacy. Studies and systematic reviews have revealed the benefit of laser therapy for acute and chronic neck pain.45 HILT can provide short-, intermediate- and long-term benefits with respect to pain control, improved range of motion and functional activity.45,53-55 HILT is also effective for the treatment of myofascial pain syndrome.56-58
Plantar Fasciitis
Laser therapy has been reported to reduce chronic heel pain arising from plantar fasciitis.6,59Both LLLT and HILT have been reported to improve pain control, functionality and quality of life in patients with plantar fasciitis.60,61 However, both the short- and long-term beneficial effects of LLLT are more variable than with HILT.59,62
Acute Postoperative Pain
About 80% of patients experience mild to severe pain after elective surgery.63 Inadequately treated pain may result in clinical and psychological changes that increase morbidity and mortality and hamper the rehabilitation process, decrease patient satisfaction regarding the overall surgical experience, and can even lead to chronic pain.64 Excessive reliance on opioid analgesics has been reported to result in prolonged opioid dependence after both major and minor surgery.65,66 In patients who have become opioid-dependent after surgery, HILT can be an effective therapy for weaning patients off chronic opioid medications.17,67-74
Acute Dental and Oral-Related Pain
Laser therapy has been demonstrated to improve orthodontic treatment by modulating the pain associated with tooth movement, preventing relapse (i.e., the tendency for teeth to return to their pretreatment position) and reducing orthodontic treatment time.75-79 Use of both LLLT and HILT has been shown to reduce soft tissue swelling, postoperative pain and the need for anti-inflammatory drugs after oral surgery.80
Mucositis, Leukoplakia and Pain Related to Radiation Therapy
Mucositis-associated pain is a major side effect of radiotherapy for head, neck and breast cancer and can lead to chronic opioid dependence. This complication impairs oral alimentation and adversely affects the nutritional state of oral surgery patients, resulting in the need to use opioid (narcotic) analgesics.81 Laser therapy has been shown to be effective in treating mucositis, leukoplakia and pain after radiotherapy in both adult and pediatric patient populations.82-94
Headache Pain
Headache is a common health problem that remains poorly responsive to pharmacologic therapies.95 Laser therapy has been applied at acupoints in the head and neck region to treat chronic headaches. The use of laser acupuncture has been reported to be a safe, noninvasive treatment for headache pain that is devoid of side effects.95-98 LLLT was effective in treating tension headaches associated with temporomandibular joint disorders, resulting in a 64% reduction in headache pain.99
Pain Due to Trigeminal Neuralgia
Trigeminal neuralgia pain, a periodic, sharp and electric shock–like facial pain, is the most common neuralgia of the head and neck region.100 The application of laser therapy can reduce the intensity of chronic neuropathic pain by increasing nerve function, promoting axonal growth and improving the capacity for myelin production in injured cranial nerves.101-107
Acute and Chronic Pain Associated With Herpes Infections
Laser therapy appears to be beneficial in improving pain control for patients with both herpes simplex and herpes zoster, as well as for recurrent herpes labialis.108 Laser treatments can also reduce the incidence of postherpetic neuralgia109 and facilitate healing of skin lesions,110 leading to a decrease in the viral titer,111 reduced level of pain and improved quality of life.112
Acute Joint and Soft Tissue Injuries
Acute muscle and soft tissue injuries are common in both weekend warriors and high-level athletes, and can lead to the loss of muscle function and result in a decreased quality of life.113,114 LLLT has been widely used as an adjuvant strategy for treatment of musculoskeletal disorders,115 to delay the onset of fatigue after exercise, to reduce the fatigue response and to improve post-exercise recovery in athletes.116-119 Laser therapy has also been shown to be efficacious for the treatment of acute lateral epicondylitis,120,121 and tendinopathies (e.g., Achilles tendon). HILT is also beneficial in treating patients suffering from acute joint injuries.
Burn-Related Pain and Skin Conditions
HILT has been effective in the treatment of post-burn pruritus, leading to an improved quality of life, decreased pain and improved functionality.122 Laser therapy has also been shown to be effective in the treatment of xanthelasma palpebrarum,123 port-wine stain124 and cutaneous leishmaniasis.125 Other skin-related superficial pain conditions that have also been successfully treated with both LLLT and HILT include genitourinary syndrome of menopause126-131 and rectovaginal fistula in patients with Crohn’s disease.132
Fibromyalgia-Related Pain
Fibromyalgia is a chronic musculoskeletal condition, characterized by widespread pain in the body, associated with particular tender points at the shoulder, back and hip region.133 A wide variety of pharmacologic drugs and dietary supplements have been used with limited success in treating the musculoskeletal pain. Laser therapy has been shown to be safe and effective in the treatment of fibromyalgia.133-135 Laser therapy promotes analgesic and anti-inflammatory effects and improves tissue healing, reduces pain and can minimize the social impact related to this common disease.136,137
Carpal Tunnel Syndrome
Carpal tunnel syndrome is one of the most common peripheral neuropathic conditions. Laser therapy has been shown to reduce pain and symptom severity, decrease sensory impairments, improve functionality, and increase hand grip strength and finger pinch muscle strength.138-148However, there are discrepancies in the literature regarding the effectiveness of LLLT in treating this common condition.149,150
Neuropathic Pain, Radiculopathy and Allodynia
Neuropathic pain is caused by a lesion or disease of the somatosensory nervous system, and epidemiological studies estimate the prevalence of neuropathic pain to be 6.9% to 10%.151Neuropathic pain is the result of abnormal processing of neuronal impulses within the central and/or peripheral nervous systems. Nerve injury often results in persistent or chronic neuropathic pain characterized by spontaneous burning pain accompanied by allodynia and/or hyperalgesia.152 Patients with neuropathic pain are commonly medicated with multiple analgesic and psychiatric medications, which increases the risk for drug–drug interactions.153 Laser therapy has proven to be clinically effective in reducing pain sensitivity,35 improving neurologic function and quality of life.154-165
Diabetic Peripheral Neuropathic Pain
According to the International Diabetes Federation in 2017, approximately 425 million adults are affected with diabetes, and many of these patients develop neuropathic pain in their extremities.166 Laser therapy has been found to be effective at improving diabetic peripheral neuropathic pain167 and improving the tissue repair process of chronic wounds in diabetic patients (e.g., diabetic foot ulcers),168,169 leading to an improved quality of life in this patient population.170
Treatment of Opioid Dependence
Opioid dependence imposes social, economic and cultural problems. In 2014, in excess of 10 million people in the United States were reportedly using prescription opioids for non-medical reasons, and approximately 2 million people met diagnostic criteria for a substance use disorder involving prescription opioids—the highest number of individuals considered to have an opioid addiction since the late 19th century.171 In a recent editorial, White suggested that nonopioid analgesics and nonpharmacologic analgesic therapies like cold laser therapy should be more extensively used for treating chronic pain rather than simply prescribing more opioid-containing medications.172 A series of 8 to 12 HILT treatments has been shown to help patients who developed opioid dependence after a major operation to discontinue their use of oral opioid-containing analgesic medications and resume their normal activities of daily living.67 Compared with other more traditional methods for treating acute and chronic pain, laser therapy has been reported to be more cost-effective and is associated with fewer side effects.173 Laser therapy also avoids the uncomfortable side effects commonly associated with opioid and nonopioid analgesics.
Contraindications and Potential Side Effects Of Laser Therapy
In contrast to pharmacotherapy, laser therapy has very few contraindications and is associated with a high degree of patient acceptance due to the absence of side effects when properly administered by a trained technician. A review by Navratil et al174 enumerated the contraindications to laser therapy: history of malignant carcinoma, irradiation of the neck region in hyperthyroidism, epileptic conditions, and direct exposure of the retina or the abdomen of a pregnant woman to the laser beam. Frigo and Lucio reported that cancer cells exposed to high dosages of laser energy (>9 J) can result in a dose-dependent increase in tumor growth, altered characteristics of collagen fibers, and can eventually lead to blood vessel growth and tumor revascularization. However, LLLT dosages (~3 J) did not alter melanoma cellular activity.175 The only well-known side effect of cold laser therapy is transient skin discoloration (redness) and a warming sensation if the hand-held laser head of a HILT device comes in close proximity to the surface of the skin (<8 in).
The current limitations to the optimal use of both LLLT and HILT for acute and chronic pain management relate to a lack of clear guidelines for optimal dose, duration and frequency of treatments when using HILT or LLLT for specific clinical disorders. Even the laser terminology (and treatment parameters) used in the peer-reviewed literature is often ambiguous. Secondly, many early clinical studies lacked a true sham-laser control group (or an active comparator group) to minimize the expected placebo effect and bias associated with using a novel analgesic therapy. Unfortunately, it is very difficult to provide a true sham treatment effect with the more powerful HILT devices.
Thirdly, many of the currently marketed laser systems differ with respect to both technical specifications and application modes (e.g., pulsed, super-pulsed, continuous). Finally, there is the substantial capital cost associated with purchasing a laser device itself (e.g., LLLT devices cost between $7,500 and $49,000 and HILT devices cost between $35,000 and $139,500). The availability of leasing programs for these devices should make them more readily available for pain clinics, surgical facilities, sports medicine clinics, rehabilitation centers and emergency departments.
The funding available to support clinical research with this novel therapeutic modality is also very limited compared with the financial support for clinical trials with new pharmaceutical agents. Given the highly questionable benefits of opioid analgesic therapy for chronic pain and the current global opioid epidemic, it would behoove organizations like the National Institutes of Health and International Association for the Study of Pain, as well as the American Academy of Pain Medicine, to start funding studies examining the role of novel nonpharmacologic therapies like cold laser therapy as an adjunct to pharmacotherapy and physical therapy in the management of acute and chronic pain.
Conclusion
Cold laser therapy is an FDA-approved, safe and effective, noninvasive, adjunctive analgesic therapy for treating acute and chronic pain. Its use is associated with high patient acceptance in the treatment of chronic pain due to its efficacy and lack of side effects. Despite its analgesic, anti-inflammatory and bio-stimulating effects, laser therapy is currently underutilized by the medical community due to a lack of knowledge regarding the benefits of this nontraditional therapy and the high cost associated with the laser device itself. Given the inherent side effects associated with both opioid and nonopioid analgesics, laser therapy should play a much more prominent role as an adjuvant for treating both acute and chronic pain.
A key factor in determining the efficacy of laser therapy with respect to providing pain relief is controlling local inflammation and facilitating wound healing. While LLLT devices with shorter wavelengths (Class I to III lasers) are more limited in their effectiveness due to their inability to deliver a sufficient number of photons to deeper tissue targets, HILT devices functioning at longer wavelengths (>1,000 nm) are able to stimulate larger areas and penetrate more deeply into soft tissues. Although both LLLT and HILT devices have been reported to be beneficial for the management of acute and chronic pain symptoms, additional clinical studies are needed to determine the optimal dose, intensity, duration, and number of the laser treatments for specific pain syndromes.
In addition, studies of the effects of laser therapy in patients with cancer, concussions and chronic degenerative neurologic diseases (e.g., chronic dementia, chronic traumatic encephalopathy, Alzheimer’s, parkinsonism) are clearly needed. The use of a combination of pharmacologic and nonpharmacologic therapies would provide a safer and more cost-effective approach to the management of pain in the future. We conclude that laser therapy could be a cost-effective adjunctive analgesic treatment for patients suffering from both acute and chronic pain.
References
- Santamato A, et al. Phys Ther. 2009;89(7):643-652.
- White PF, et al. F1000Res. 2017;6:2161.
- Nakamura T, et al. Laser Ther. 2014;23(4):273-277.
- Youssef EF, et al. J Lasers Med Sci. 2016;7(2):112-119.
- Coca KP, et al. Pain Manag Nurs. 2016;17(4):281-289.
- Macias DM, et al. J Foot Ankle Surg. 2015;54(5):768-772.
- Posten W, et al. Dermatol Surg. 2005;31(3):334-340.
- Alayat MS, et al. Lasers Med Sci. 2014;29(1):335-342.
- Kheshie AR, et al. Lasers Med Sci. 2014;29(4):1371-1376.
- Zati A, et al. J Biol Regul Homeost Agents. 2012;26(4):701-711.
- Kim SH, et al. Man Ther. 2015;20(6):751-757.
- Alayat MS, et al. Lasers Med Sci. 2017;32(3):503-511.
- Dundar U, et al. Lasers Med Sci. 2015;30(3):1097-1107.
- Alayat MS, et al. Lasers Med Sci. 2014;29(3):1065-1073.
- De Freitas LF, Hamblin MR. IEEE J Sel Top Quantum Electron. 2016;22(3). pii: 7000417.
- Karu T. J Photochem Photobiol B. 1999;49(1):1-17.
- Karlekar A, et al. Ann Card Anaesth. 2015;18(3):317-322.
- Alghadir A, et al. Lasers Med Sci. 2014;29(2):749-755.
- Fukuda VO, et al. Rev Bras Ortop. 2015;46(5):526-533.
- Huang YY, et al. Dose Response. 2009;7(4):358-383.
- Chow R, et al. Photomed Laser Surg. 2011;29(6):365-381.
- Chow RT, Armati PJ. Photomed Laser Surg. 2016;34(12):599-609.
- Fulop AM, et al. Clin J Pain. 2010;26(8):729-736.
- Enwemeka CS. Photomed Laser Surg. 2009;27(3):387-393.
- Bjordal JM, Baxter GD. Photomed Laser Surg. 2006;24(4):533-534.
- Huang Z, et al. Arthritis Res Ther. 2015;17:360.
- Joensen J, et al. Photomed Laser Surg. 2012;30(12):688-694.
- Tunér J, Hode L. J Clin Laser Med Surg. 1998;16(5):245-248.
- Hode L, Tunér J. Lasers Surg Med. 2006;38(4):343.
- Tunér J, Hode L. Clin Rheumatol. 2010;29(9):1075-1076.
- Tunér J, Hode L. ISRN Otolaryngol. 2013;2013:839256.
- Enwemeka CS, et al. Photomed Laser Surg. 2004;22(4):323-329.
- Reddy GK. J Clin Laser Med Surg. 2004;22(2):141-150.
- Gross AR, et al. Open Orthop J. 2013;7:396-419.
- De Andrade AL, et al. J Photochem Photobiol B. 2016;164:36-42.
- www.thorlaser.com/?courses/?index-US.php. Accessed September 5, 2019.
- Kim GJ, et al. J Phys Ther Sci. 2016;28(11):3197-3199.
- Viliani T, et al. Energy for Health. 2012;9:18-22.
- Angelova A, Ilieva EM. Pain Res Manag. 2016;2016:9163618.
- Ip D. Lasers Med Sci. 2015;30(9):2335-2339.
- White PF, et al. J Mol Biomark Diagn. 8:343. doi: 10.4172/2155-9929.1000343.
- Meireles SM, et al. Clin Rheumatol. 2010;29(5):501-509.
- El-Shamy SM, Abdelaal AAM. Disabil Rehabil. 2018;40(4):462-468.
- Demartis F, et al. Energy for Health. 2013;11:4-8.
- Yousefi-Nooraie R, et al. Cochrane Database Syst Rev. 2008;(2):CD005107.
- Jackson T, et al. Anesth Analg. 2016;123(3):739-748.
- Basford JR, et al. Arch Phys Med Rehabil. 1999;80(6):647-652.
- Fiore P, et al. Eur J Phys Rehabil Med. 2011;47(3):367-373.
- Kolu E, et al. Pak J Med Sci. 2018;34(3):530-534.
- Ay S, et al. Clin Rheumatol. 2010;29(8):905-910.
- Chou R, Huffman L. Guideline for the evaluation and management of low back pain: evidence review. Glenview IL: American Pain Society, 2007.
- Taradaj J, et al. Clin Interv Aging. 2018;13:1445-1455.
- Chow RT, et al. Lancet. 2009;374(9705):1897-1908.
- Alayat MS, et al. Lasers Med Sci. 2016;31(4):687-694.
- Haladaj R, et al. Med Sci Monit. 2017;23:335-342.
- Carrasco TG, et al. Cranio. 2009;27(4):243-247.
- Dundar U, et al. Lasers Med Sci. 2015;30(1):325-332.
- Konstantinovic LM, et al. Pain Med. 2010;11(8):1169-1178.
- Jastifer JR, et al. Foot Ankle Int. 2014;35(6):566-571.
- Ordahan B, et al. Lasers Med Sci. 2018;33(6):1363-1369.
- Dos Santos SA, et al. Photobiomodul Photomed Laser Surg. 2019;37(6):327-335.
- Basford JR, et al. Arch Phys Med Rehabil. 1998;79(3):249-254.
- Apfelbaum JL, et al. Anesth Analg. 2003;97(2):534-540.
- Lamplot JD, et al. J Arthroplasty. 2014;29(2):329-334.
- Goesling J, et al. Pain. 2016;157(6):1259-1265.
- Alam A, et al. Arch Intern Med. 2012;172(5):425-430.
- White PF, et al. J Clin Anesth. 2017;40:51-53.
- Ezzat AE, et al. Int J Pediatr Otorhinolaryngol. 2016;89:183-186.
- Kreisler MB, et al. Int J Oral Maxillofac Surg. 2004;33(1):38-41.
- Jackson R, et al. Am J Cosmet Surg. 2009;26:144-148.
- Moore KC, et al. Laser Therapy. 1992;4(4):145-149.
- Nesioonpour S, et al. Anesth Pain Med. 2014;4(3):e17350.
- Langella LG, et al. Lasers Med Sci. 2018;33(9):1933-1940.
- Fernandes GA, et al. Ann Card Anaesth. 2017;20(1):52-56.
- Sonesson M, et al. BMC Oral Health. 2016;17(1):11.
- Almallah MM, et al. J Clin Diagn Res. 2016;10(11):ZC23-ZC28.
- Artés-Ribas M, et al. Lasers Med Sci. 2013;28(1):335-341.
- Sousa MV, et al. Photomed Laser Surg. 2014;32(11):592-599.
- Yang H, et al. Photobiomodul Photomed Laser Surg. 2019;37(6):349-355.
- Mallick S, et al. Eur Arch Otorhinolaryngol. 2016;273(9):2285-2293.
- Markovic AB, Todorovic L. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):e4-e8.
- Gautam AP, et al. Radiother Oncol. 2012;104(3):349-354.
- Robijns J, et al. Lasers Med Sci. 2017;32(1):229-242.
- Carvalho PA, et al. Oral Oncol. 2011;47(12):1176-1181.
- Lima AG, et al. Braz Dent J. 2010;21(3):186-192.
- Oberoi S, et al. PLoS One. 2014;9(9):e107418.
- Soares RG, et al. Lasers Med Sci. 2018;33(8):1813-1819.
- Bjordal JM, et al. Support Care Cancer. 2011;19(8):1069-1077.
- He M, et al. Eur J Pediatr. 2018;177(1):7-17.
- Amadori F, et al. Lasers Med Sci. 2016;31(6):1231-1236.
- Gautam AP, et al. Support Care Cancer. 2013;21(5):1421-1428.
- Antunes HS, et al. Radiother Oncol. 2013;109(2):297-302.
- Matulic N, et al. Photobiomodul Photomed Laser Surg. 2019;37(6):362-368.
- Bernal Rodriguez CG, et al. Photobiomodul Photomed Laser Surg. 2019;37(4):240-243.
- Ebneshahidi NS, et al. Acupunct Med. 2005;23(1):13-18.
- Gottschling S, et al. Pain. 2008;137(2):405-412.
- Skelly AC, et al. Rockville (MD): Agency for Healthcare Research and Quality (US). 2018 Jun. Report No.: 18-EHC013-EF.
- King CE, et al. Phys Ther. 1990;70(1):24-30.
- Tomaz de Magalhães M, et al. Exp Biol Med (Maywood). 2016;241(1):40-44.
- Falaki F, et al. J Dent Res Dent Clin Dent Prospects. 2014;8(1):1-5.
- Costantini D, et al. Ann Ital Chir. 1997;68(4):505-509.
- Pinheiro AL, et al. J Clin Laser Med Surg. 1998;16(4): 223-226.
- Iijima K, et al. Clin J Pain. 1989;5(3):271-274.
- Walker J, et al. Clin J Pain. 1987;3(4):183-187.
- Ebrahimi H, et al. J Lasers Med Sci. 2018 Winter;9(1):63-68.
- Pinheiro ALB, et al. Proc. SPIE 10048, Mechanisms of Photobiomodulation Therapy XII, 1004808
- de Pedro M, et al. J Oral Facial Pain Headache. 2019 Jul 24 [Epub ahead of print].
- de Paula Eduardo C, et al. Lasers Med Sci. 2014;29(4):1517-1529.
- Fan X, Wang S. Dermatol Surg. 2015;41(10):1189-1190.
- Chen YT, et al. J Am Acad Dermatol. 2016;75(3):572-577.
- Zverev VV, et al. Lasers Med Sci. 2016;31(5):849-855.
- Gomes RNS, et al. Rev Bras Geriatr Gerontol. 2018; 21(1). http://dx.doi.org/?10.1590/?1981-22562018021.170116
- Leal-Junior EC, et al. Lasers Med Sci. 2015;30(2):925-939.
- Sundstrup E, et al. Eur J Pain. 2017;21(2):366-373.
- Foley J, et al. Laser Ther. 2016;25(1):35-42.
- Leal Junior EC, et al. Photomed Laser Surg. 2008;26(5):419-424.
- Leal Junior EC, et al. J Orthop Sports Phys Ther. 2010;40(8):524-532.
- Baroni BM, et al. Eur J Appl Physiol. 2010;110(4):789-796.
- Baroni BM, et al. Photomed Laser Surg. 2010;28(5):653-658.
- Bjordal JM, et al. BMC Musculoskelet Disord. 2008;9:75.
- Celik D, Anaforoglu Kulunkoglu B. Photobiomodul Photomed Laser Surg. 2019;37(5):269-275.
- Ebid AA, et al. Lasers Med Sci. 2017;32(3):693-701.
- Li D, et al. Photobiomodul Photomed Laser Surg. 2019;37(4):244-247.
- Ma G, et al. Photobiomodul Photomed Laser Surg. 2019;37(6):376-380.
- Silva MLF, et al. Photobiomodul Photomed Laser Surg. 2019;37(5):298-304.
- Lanzafame RJ, et al. Photobiomodul Photomed Laser Surg. 2019;37(7):395-407.
- Tadir Y, et al. Lasers Surg Med. 2017;49(2):137-159.
- Kwon TR, et al. Lasers Surg Med. 2018;50(9):940-947.
- Hardy LA, et al. Lasers Surg Med. 2017;49(2):198-205.
- Bashkatov AN, et al. J Appl Phys D. 2005;38(15):2543.
- Enwemeka CS. Laser Ther. 2001;13:95-101.
- Drumond DG, et al. Photobiomodul Photomed Laser Surg. 2019;37(7):451-454.
- White PF, et al. Rheumatol Int. 2018;38(3):517-523.
- Gur A, et al. Rheumatol Int. 2002;22(5):188-193.
- Gur A, et al. Lasers Med Sci. 2002;17(1):57-61.
- Germano Maciel D, et al. Lasers Med Sci. 2018;33(9):1949-1959.
- de Souza RC, et al. Med Oral Patol Oral Cir Bucal. 2018;23(1):e65-e71.
- Shooshtari SM, et al. Electromyogr Clin Neurophysiol. 2008;48(5):229-231.
- Chang WD, et al. Photomed Laser Surg. 2008;26(6):551-557.
- Dincer U, et al. Photomed Laser Surg. 2009;27(1):119-125.
- Alves MP, de Araujo GC. Rev Bras Ortop. 2015;46(6):697-701.
- Lazovic M, et al. Photomed Laser Surg. 2014;32(6):336-344.
- Jiang JA, et al. J Phys Ther Sci. 2011;23(4):661-665.
- Fusakul Y, et al. Lasers Med Sci. 2014;29(3):1279-1287.
- Bartkowiak Z, et al. Indian J Orthop. 2019;53(2):347-352.
- Ahmed OF, et al. BBA Clin. 2017;8:43-47.
- Tezcan S, et al. J Ultrasound Med. 2019;38(1):113-122.
- Güner A, et al. Rheumatol Int. 2018;38(5):895-904.
- Burger M, et al. Physiother Theory Pract. 2017;33(3):184-197.
- Bekhet AH, et al. Lasers Med Sci. 2017;32(6):1439-1448.
- van Hecke O, et al. Pain. 2014;155(4):654-662.
- Oliveira ME, et al. Photochem Photobiol Sci. 2017;16(4):547-554.
- Pickering G, et al. Drugs Aging. 2016;33(8):575-583.
- Rochkind S, et al. Photomed Laser Surg. 2007;25(5):436-442.
- Khullar SM, et al. Eur J Oral Sci. 1995;103(5):299-305.
- Câmara CN, et al. Acta Cir Bras. 2011;26(1):12-18.
- dos Reis FA, et al. Lasers Med Sci. 2009;24(5):741-747.
- Chen YS, et al. Microsurgery. 2005;25(1):83-89.
- Barbosa RI, et al. Lasers Med Sci. 2010;25(3):423-430.
- Medalha CC, et al. Lasers Med Sci. 2012;27(3):629-635.
- Shen CC, et al. J Biomed Mater Res A. 2013;101(1):239-252.
- Mohammed IF, et al. Photomed Laser Surg. 2007;25(2):107-111.
- Hüllemann P, et al. Eur J Pain. 2017;21(5):918-926.
- Führer-Valdivia A, et al. Med Oral Patol Oral Cir Bucal. 2014;19(4):e327-e334.
- Fallah A, et al. Lasers Med Sci. 2017;32(3):721-728.
- International Diabetes Federation. IDF Diabetes Atlas 8th ed. (2017). idf.org/?e-library/?epidemiology-research/?diabetes-atlas.html. Accessed September 5, 2019.
- Hazari A, et al. J Cosmet Laser Ther. 2017;19(6):360-363.
- de Alencar Fonseca Santos J, et al. Photomed Laser Surg. 2018;36(6):298-304.
- Mathur RK, et al. Lasers Med Sci. 2017;32(2):275-282.
- Anju M, et al. Diabetes Metab Syndr. 2019;13(2):1087-1091.
- Dowell D, et al. JAMA. 2016;315(15):1624-1645.
- White PF. Expert Opin Pharmacother. 2017;18(4):329-333.
- Ebid AA, El-Sodany AM. Lasers Med Sci. 2015;30(6):1747-1755.
- Navratil L, Kymplova J. J Clin Laser Med Surg. 2002;20(6):341-343.
- Frigo L, et al. Lasers Med Sci. 2018;33(6):1215-1223.









Leave a Reply
You must be logged in to post a comment.