Anesthesiology News
This year Felipe Urdaneta, MD, the president-elect of the Society for Airway Management (SAM), presented seven questions to an international panel of airway experts. The panelists were chosen by Dr. Urdaneta, who also answered the questions.
1.What two main components would you modify from the 2013 American Society of Anesthesiologists (ASA) difficult airway guidelines?1
Dr. Foley: Jet ventilation is one main issue I would like to address and see modified in the ASA airway guidelines. Jet ventilation should be removed as one of the invasive airway techniques. Narrow-bore cricothyrotomy requiring high-pressure jet ventilation with a high failure rate has been reported in the Fourth National Audit Project (NAP4)2 and ASA Closed Claims Project.3High-pressure jet ventilation can cause stacking and barotrauma. The catheter can become kinked and, if dislodged, cause subcutaneous emphysema, making it difficult to do a surgical airway. The small-bore cannula has no cuff, and is therefore unable to provide airway protection, placing the patient at higher risk for aspiration.
Dr. Galgon: Two issues in the current ASA airway guidelines that I would modify include:
- placing an increased emphasis on first-attempt optimization, and
- increasing the emphasis on parallel versus linear management.
Optimizing first attempts, whether at the level of awake versus asleep airway management, or bag-mask ventilation, or intubation, increases airway management efficiency and offers a more rapid movement to rescue techniques when needed. First-attempt optimization can include multiple factors, such as patient positioning, immediate use of airway management adjuncts (e.g., an oral airway or high-flow nasal cannula [HFNC] apneic oxygenation), and matching technology to the patient or situational characteristics. We know that failure to act—or decision to act—is a significant contributor when serious injuries related to airway management are reviewed. First-attempt optimization provides more efficient diagnostic information regarding the likelihood for success of an attempt and then movement to a follow-on step in the event of difficulty or failure of the attempt.
In a similar manner, parallel management (i.e., preparing for the next step or two in airway management) increases efficiency with movement toward an effective rescue technique. This type of thinking is illustrated in the Vortex approach to airway management.4
Dr. Law: I like the ASA guidelines and suspect they have helped prevent a lot of airway-related morbidity and mortality. The first of two things I might try to modify relates to the algorithm. While the messages contained in the algorithm are good, it is a bit difficult to reproduce them from memory. So, while retaining the messages, the algorithm needs to be simplified to make it more easily retrieved from memory when the clinician is in a difficult situation.
Second, we need more guidance on who needs awake tracheal intubation. We have all successfully managed the airways of patients with predictors of difficulty after the induction of general anesthesia—or should I say, “We’ve all gotten away with?” But many guidelines are silent on when this falls within the standard of care as safe practice or when it does not. In my opinion, it is no longer sufficient to simply advise “consideration of the relative clinical merits and feasibility … of awake intubation versus intubation after induction of general anesthesia.”1 Rather, even if only expert consensus–based, we need more guidance on safe decision making when difficulty is predicted.
Dr. Mir: The two main modifications that I would like to suggest in the ASA guidelines are:
- They are very comprehensive and cover all eventualities. This approach is great in a non–emergency situation where one has a lot of time at hand. However, in a critical emergency, such as a failed airway, this cognitive aid may fail to provide the immediate guidance to the anesthesiologist who may be stressed and unable to identify the relevant part of the guidelines with a quick look. So I would limit them to emergency airway management only, that is, face mask, supraglottic airway device (SAD), and front-of-neck access (FONA; at the bottom of the guidelines page).
- The attempt to return to spontaneous ventilation may be detrimental. This is because the muscle relaxant may be reversed easily, but the sedative (e.g., propofol) may still cause airway obstruction. So this may lead to a situation where ensuring muscle paralysis may be a better option, as it gives the anesthesiologist full control of manual ventilation and provides the best conditions for that.
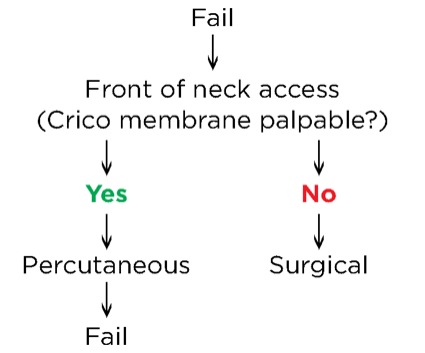
Dr. Perin: First, it is the algorithm that I use in my daily practice. I believe it is the most direct and clear, with all the tools that you need to make your management choices. The first thing I would modify is the term “emergency invasive airway access.” It would be better to say “front-of-neck access,” because this gives you a quicker idea of what you should do. I would add two arrows to indicate whether you palpate the cricothyroid membrane to decide if you proceed with a percutaneous or surgical technique (Figure).
The other thing I would suggest changing would be mention of “initial intubation attempts.” What one considers multiple attempts might be different from what others consider multiple attempts; what is the magic number? In my opinion we should change this and incorporate the phrase “best attempt” with an explanation of what is a best attempt at intubation, highlighting position, preoxygenation, use of a malleable stylet, and size of the device, among other things, all of which are very important variables to determine a true best attempt.
Dr. Straker: I would like to see the use of noninvasive nasal continuous positive airway pressure (PAP) devices specifically added to the guidelines, rather than the generality of “actively pursue opportunities to deliver supplemental oxygen throughout the process of difficult airway management.” Consider preoxygenation with HFNC routinely rather than a traditional mask. If the intubation proves difficult, you have the advantage of HFNC already in place and can maintain a longer period of apnea without a rapid drop in oxygen saturation.
Dr. Urdaneta: I consider the ASA guidelines to be the backbone and gold standard of all other available airway guidelines. They are comprehensive and include all phases of airway management, including preoperative evaluation. In order to understand them better and to answer this question, I think we need to divide the guidelines into technical and nontechnical aspects of airway management.
I am going to take the liberty to discuss two factors of each of these that I believe should be modified for future versions of the guidelines. For nontechnical factors, I believe the guidelines should be simplified. The one criticism I always hear about them is the fact that, overall, there are too many steps and options that can lead to information overload and contribute to nonadherence, and perhaps failure; therefore, I would streamline them and only have a limited set of options, and each option should have only two choices (yes or no) in order to have a clearer path to follow in case difficulty arises. The other nontechnical aspect I would modify is that extubation is named, but no guideline exists regarding this important phase of airway management.
About technical factors, I believe there needs to be a clearer picture regarding invasive options; specifically, I question whether in this day and age there is a role for the use of jet ventilation and retrograde intubation techniques in urgent/emergent cases. The other technical issue I would modify is the issue of pharmacology and airway management. I believe the guidelines should address pharmacology, not just of hypnotics and sedatives but also regarding the use of muscle relaxants. We just talk about “sleep” and “awake” states, but this is not sufficient.
Dr. Valencia Orgaz: In my opinion, the two main changes that need to be introduced in any future ASA airway management guidelines are:
- Redesign the structure in order to allow approach by phases/stages, establishing clear pathways from one phase to the next, so that it can be used as a cognitive aid in times of crisis.
- Establish a clear recommendation on how to approach patients with questionable airways. By that, I mean when we find certain predictors of difficult laryngoscopy and have doubts whether ventilation would be optimal via bag-mask ventilation or an SAD. Using a conservative approach, these people should be approached as patients with difficult airways and managed either with awake or spontaneous ventilation. There are colleagues who consider labeling them as “difficult airway” patients condemns them to an awake approach for life.
Dr. Wong: My first suggestion is about the emergent airway. An emergency pathway is declared after failure to 1) intubate, 2) face-mask ventilate, or 3) rescue with a laryngeal mask (LM) airway. I believe it is important to add a dose of muscle relaxant to overcome potential laryngospasm or a patient resisting airway insertion prior to a surgical airway. My second suggestion pertains to “emergency invasive airway access.” The current favored terminology is FONA. As for the FONA technique, the upcoming Canadian airway guidelines, which are based on consensus, recommend an open scalpel bougie technique rather than leaving readers with the three to four options currently stated in the ASA algorithm.
2.In the era of video laryngoscopy (VL), how do you teach and keep your own skills up-to-date with regard to flexible endoscopic intubation?
Dr. Foley: As a private practitioner, if I consider a patient a possible difficult airway, I will do an awake flexible fiber-optic bronchoscopy (FFB) intubation. Otherwise, since I teach difficult airway courses, I would practice the fiber-optic scope. When I was in academics, I would teach FFB intubations to residents when the patient was under general anesthesia and required an endotracheal tube (ETT). My recommendation is to attend an airway workshop at least once a year.
Dr. Galgon: Being an academic anesthesiologist, not only do I have to maintain my own skills, but I also accept the obligation of teaching residents how to perform flexible endoscopic intubations. It is true that the introduction and acceptance of VL has greatly reduced the use of flexible endoscopic intubation. To achieve the two goals noted above, I encourage 1) practice using airway management trainers, which help to build and maintain basic equipment familiarity and manual dexterity skills, and 2) a low threshold for performing flexible endoscopic intubation in situations where it is indicated (e.g., in the presence of cervical spine pathology), whereas some of my colleagues may reasonably choose a first attempt with VL. I also have the fortunate opportunity to continue to conduct clinical studies in airway management that often involve the use and navigation of a flexible bronchoscope through the airway.
Dr. Law: I maintain my own skills in a number of ways: First, I use the flexible endoscope for all of my awake intubations, that is, despite the reported efficacy of VL for some awake intubations.5Second, I default to a combination of a flexible endoscope with VL or through an SAD if I have failed to intubate the patient with direct laryngoscopy or VL on its own. Third, I continue to use flexible endoscopy for the many other uses in anesthetic practice that have not gone away (e.g., double-lumen tube positioning, ruling out mainstem intubation if unclear, addressing tracheal soiling or mucous plugging, etc.).
I also teach quite a few airway courses around the country, and at every course I do up to 50 flexible endoscopic intubations on a mannequin during coffee breaks to help maintain my skills with the device. Regarding teaching others, I am fortunate to work in an academic center and regularly supervise trainees in performing awake as well as post-induction flexible endoscopic intubations in fasted, elective surgical patients. In the latter group, I do this with the residents simply for skills acquisition purposes.
Dr. Mir: I look for opportunities to perform flexible endoscopic intubations and perform asleep flexible endoscopic intubations where possible. This keeps up my flexible scope maneuvering skills. I also try to attend the outpatient ENT (ear, nose and throat) clinic with my surgical colleagues and encourage my trainees to go there—sometimes during a long case in the surgical theater—to practice and learn flexible endoscopic skills.
Dr. Perin: This is a big issue nowadays; everyone needs to understand that FFB offers several advantages over other devices that makes it mandatory to learn and master. In my opinion, the best way to teach and keep these skills involves two steps:
- First step (learning skills): Review your knowledge of the device (lectures, literature, and meetings), and then practice in low-fidelity mannequins and fiber-optic simulators, because these can teach you airway navigation and how to overcome obstacles.
- Second step (clinical skills): Start doing regular elective cases using an asleep technique with the aid of someone more skilled than you, and use the bronchoscope to navigate through an SAD to be more confident of airway anatomy. Finally, do your difficult awake cases with the aid of someone more skilled than you, and in the end, do your difficult cases alone. (Do not forget that in difficult cases, help is always a better option to have.)
Dr. Straker: I run a yearlong combined airway/ENT fellowship and a one-month airway rotation at my institution. The residents and fellows are only allowed to use VL as a terminal rescue. Fiber-optic training is actively taught as a first-line intubation technique, followed by all other devices during the rotation.
Dr. Urdaneta: It is inevitable that in the era of VL, the role and numbers of endoscopic intubations have been affected. The way I tackle this issue is to follow the ASA algorithm: If I find any reason or if my “spider sense” gives me a compelling reason that patient X should be done awake, I do not hesitate to perform an endoscopic intubation. Having said this, because VL very often will suffice, I find myself performing more sleep endoscopic intubations for teaching purposes. I also encourage those who are interested that they should plan to attend an airway course at least yearly where some seasoned and expert instructors can give them invaluable tips and tricks that can help them become more comfortable with the art of flexible intubation.
Dr. Valencia Orgaz: Acquiring and maintaining skills with flexible endoscopic intubation is an essential—and I would say gold standard—principle for the management of a patient with a difficult airway. Flexible endoscopic intubation can be used when it is planned or during rescue attempts, for example, when intubating via an SAD.
We recommend that all our trainees take a four-week airway management rotation that I coordinate, in which participants get to perform endoscopic intubations with asleep and awake patients and via the oral and nasal routes. They also work alongside pulmonologists in their bronchoscopy laboratory so they become comfortable with the use of flexible endoscopic techniques.
Dr. Wong: First, for suspected difficult airway cases, I frequently do asleep FFB intubation for teaching and maintenance of competence. An airway operator will not be proficient in FFB if he/she only does it in rare airway crisis situations. Second, many airway operators are losing their awake FFB skills due to dependence on video laryngoscopic intubation. Personally, for other high-risk difficult airways (e.g., cervical spine pathology or morbid obesity), I have a low threshold for doing an awake FFB.
3.Do you have an airway lead program in your practice? Do you consider this a priority for your practice?
Dr. Foley: Yes, we have an airway lead program, as I am the airway lead person at my hospital. I believe it is very important to have an airway lead person in each hospital just as you would have a lead person for any other subspecialty. Major patient morbidity and mortality occur during difficult airway management in the OR, ICU and emergency department (ED), as reported by ASA Closed Claims analysis and NAP4.2,3 It was suggested in the NAP4 report that there should be an airway lead person.
The airway lead should manage the standardization of airway equipment and keep up-to-date with new technology, techniques, and education in airway management. The airway lead would be responsible for communicating that the patient has a difficult airway through an in-hospital registry, if possible, as well as a national registry, such as MedicAlert.
Dr. Galgon: Although we have several airway experts in our group practice to whom colleagues turn for help and knowledge, we do not have an airway lead program per se, but I believe this is a great idea. In my opinion, a leader in such a program does not have to be an airway expert per se, but should be an individual with an interest in airway management. Such a program would provide this leader with the support and resources to organize an institution’s 1) policies and procedures, 2) equipment, 3) personnel resources, and 4) educational and training activities to emphasize the importance of airway management and improve its practice within the institution.
Dr. Law: The airway lead is a concept recommended and endorsed by the United Kingdom’s Difficult Airway Society (DAS), the Royal College of Anaesthetists (RCoA), and the NAP4 report.6The airway lead role includes overseeing local training in airway management, ensuring policies are in place for airway emergencies, auditing local airway practice, ensuring equipment availability, etc.
We do not have an airway lead program in our practice. We fulfill many of the roles advocated for an airway lead, but in a more ad hoc fashion. Although perhaps not ideal, we are helped in this regard by having a significant number of airway enthusiasts, educators, and researchers in our department. Notwithstanding, I would like to see the concept of the formal airway lead cross the pond and become more entrenched in North America; it could only help the cause of safe airway management.
Dr. Mir: We have had a national Airway Leads Program in the United Kingdom since 2012, which was set up after the NAP4 report was published. The NAP4 report recommended an airway lead in every hospital/trust, and the RCoA and DAS fully support this role. This role is included in individual job plans and recognized by all trusts. I strongly believe this is essential for the development and maintenance of airway skills among anesthesiologists. The airway lead acts as a link between the RCoA and individual anesthesiologists. The airway lead is responsible for developing and implementing policies, ensuring airway training within the department, and standardizing difficult airway equipment within the trust in accordance with the local guidelines and recommendations from DAS.
Dr. Perin: At my hospital, I have a rapid response team for airway management, with anesthesiologists and intensivists, and the team covers the ED, ORs and ICUs. Concerning priority, I think that it is good to have highly skilled personnel to help with airway management, but they cannot be everywhere at every time. As an example, there was a patient with a difficult airway who was in the ICU, and the intensivist involved in the case did not remember to call the response team.
We should invest time by training staff technical and especially nontechnical skills. The training techniques will vary depending on the type of hospital, type and number of providers involved with airway management, and type of resources available at each institution.
Dr. Straker: By default, I have become the airway lead in my department. It is not a formalized process. I just have a keen interest in airway management; a good relationship with the otorhinolaryngology, critical care, and ED services; and run the combined airway/ENT fellowship. I do not consider this a priority in my practice, but it is nice to have a point person within the department who works well with those other services that typically see challenging airways, coordinate equipment, and formalize a consult service.
Dr. Urdaneta: This is an initiative from our DAS colleagues and something they have recommended since the aftermath of NAP4. Do I think it makes sense? Absolutely, it makes sense, as essentially it ensures there is a point/contact person who is the champion in airway matters and can work on getting airway policies, procedures, equipment, and training into each hospital.
Having said this, unfortunately, this initiative has not been universally adopted in the United States. Although I do not have any formal numbers, I believe most of us carry the airway lead’s duties but in an informal capacity, which is less than ideal. Perhaps we need strong endorsement and partnerships between organizations, such as SAM and the Anesthesia Patient Safety Foundation, to spread the word and increase the number of institutions and organizations with a formal airway lead.
Dr. Valencia Orgaz: As I mentioned in the previous question, one of our main goals is education and training. I run a monthlong rotation on difficult airway management, but this is mainly for trainees. I think this is a fundamental service we provide, and I wish we had more time to train all anesthesia staff in advanced airway management. Those who cannot participate in our rotation or our graduated colleagues have to attend external courses.
Dr. Wong: At our two hospital sites, we have several anesthesiologists who have special interests in airway management. But we don’t have an airway lead, so to say. In order to do multicenter prospective studies, it is important to have airway leads, as seen in the NAP4 study.
4.After a high-risk extubation with the use of an airway exchange catheter (AEC), how long do you recommend keeping the AEC in place?
Dr. Foley: The length of time I would keep the AEC in place would depend on the clinical situation. Is it someone who has an anatomically difficult airway before surgery or more of a physiologic difficult airway after surgery, with edema, trauma, or other clinical factors that may influence respiratory failure requiring reintubation? The length of time might range from two to 12 hours. Most patients tend to tolerate the AEC.
Dr. Galgon: I generally attempt to maintain the AEC in situ to a time point beyond which the risks for respiratory failure and/or upper airway obstruction (eg, from airway management or surgery-related upper airway edema) have sufficiently dissipated. The risk associated with periglottic edema generally dissipates within 15 to 60 minutes post-extubation, while the risk for respiratory failure or surgical edema can generally persist for 12 to 24 hours post-extubation.
The two largest studies regarding the use of an AEC to maintain post-extubation airway access include that by Mort7 and Roten et al.8 Both of these studies have demonstrated an increase in reintubation success and reduction in associated complication rates when an AEC was in situ and used for reintubation. The challenge is always the patient’s tolerance of an AEC. In the study by Mort, patients tolerated an AEC for a mean time of 3.9 hours (range, five minutes to 72 hours). Smaller (11-14 Fr) catheters were better tolerated than larger (19 Fr) catheters.7 The study by Roten et al demonstrated that nasal AECs were better tolerated than oral AECs, with no difference in tracheal displacement rates.8
Besides placement route and size, one additional point to consider improving patient tolerance is insertion depth. I try to ensure the distal tip of the AEC is in the mid- to distal trachea to maintain an intratracheal position, but sufficiently above the carina to reduce the possibility of coughing. This can be accomplished by using the depth markers on the AEC.
Dr. Law: Perhaps glib, but my answer to this one is, “It depends what you’re worried about.” As an anesthesiologist, when I leave in a placeholder AEC upon extubation, most often it is due to a likely difficult reintubation plus concern that the patient might not maintain airway patency upon extubation, as might happen with significant airway edema. In this situation, upon extubation, if the airway indeed obstructs, I am going to know about it pretty well right away. If the patient is fine and does not require reintubation, I leave the AEC in for only a short period of time (e.g., no more than 15-30 minutes). On the other hand, an ICU patient may prove intolerant of extubation for a variety of reasons, for example, due to failed gas exchange some hours later despite maintaining a patent upper airway. Thus, for the ICU patient suspected or known to be difficult to reintubate, when used, the placeholder AEC should be left in correspondingly longer. In a 2007 study on AEC use—mostly in ICU patients—21 of 51 patients were reintubated over an AEC within two hours, and the remaining 59% between two and 10 hours after initial extubation.7
Dr. Mir: This is a case-dependent decision. I generally keep the AEC in place until the patient’s airway is considered safe, usually after a few hours of recovery, until the time when the patient is fully awake and conscious.
Dr. Perin: I think this question depends on the patient’s condition. The first question for a high-risk extubation is, “Should I proceed or wait some more time?” Thomas C. Mort, MD, once said, “Just because you can, doesn’t mean you should extubate.” If you think you can proceed, then the use of an AEC is highly recommended.
Because extubation failure can occur up to 72 hours after extubation, I leave the regular AEC in at least 24 hours. A newer soft tip and thin-wire AEC is less traumatic and therefore can be used for longer periods of time. For certain cases, I recommend a new catheter (Cook Staged Extubation Set; Cook Medical) should remain in place up to 48 to 72 hours after extubation, when there is concern for a delayed need for reintubation.
Dr. Straker: The literature states that these catheters can be left in situ up to 72 hours. I keep the AEC in place until I am confident, based on objective criteria (vital signs and physical signs, eg, retractions, tracheal tugging, etc), that the patient is able to maintain his/her airway, wide awake, and free of pain and comfortable.
Dr. Urdaneta: The concerns, causes, and adverse events from extubation, including extubation failures, although grouped together for the purpose of discussion, should be individualized based on the patient, the condition, and surgical factors. Elective extubation of high-risk patients and procedures can take place in the OR and/or PACU, as well as in the ICU. Extubation-related adverse events can be immediate or early (within one to two hours after extubation), or late, meaning after 24 hours. I am of the opinion that AECs play a role in the early post-extubation stages, but not in the late ones.
We do not have a rigid protocol regarding duration of use of an AEC. Most extubation issues happen early, within one to two hours, and therefore we usually keep the catheter for a maximum of two hours. I find our AECs cannot be tolerated longer and are awkward to secure. Truth be told, most of my patients, and the staff taking care of them, do not tolerate the AEC for more than one hour. Unfortunately, I do not have access to the newer staged extubation sets, which seem to be better tolerated for longer periods of time and are easier to secure.
Dr. Valencia Orgaz: It really depends on specific circumstances:
- Was the difficulty due to a previous condition, such as a known difficult airway, or for other reasons, such as obesity or obstructive sleep apnea?
- Did something happen during the perioperative period, such as airway edema, hematoma, or distortion of the pharyngeal-laryngeal-tracheal axis?
- Is there a new issue that limits access to the airway (i.e., tracheal resection)?
In the first case, we maintain the AEC only until the patient responds to commands, is able to protect the airway and control secretions, and there is hemodynamic and respiratory stability. In the other two scenarios, we do not have an established maximum time of use of the AEC. In some instances, an AEC has been well tolerated for more than 72 hours, with minimal sedation and remifentanil or dexmedetomidine, if needed.
Dr. Wong: As opposed to intubation, extubation is always elective. For intermediate-risk cases, an AEC may be quite useful for reintubation. From my experience, a fully awake patient with intact neuromuscular function simply will not tolerate an AEC in situ. It will tickle the trachea when the patient turns his head, leading to irritation or coughing. I found instilling lidocaine 2% using a MAD Nasal atomizer (Teleflex) via an ETT before AEC deployment and extubation allows AEC tolerance for a longer period. I recommend keeping the AEC in situ until the patient resists the AEC or 30 to 60 minutes have elapsed. This is the highest risk period for reintubation.
5.A patient with a suspected difficult intubation in the past (had an unplanned admission to the ICU, was kept on a ventilator overnight, and remembers a severe sore throat afterward), but no further documentation or information available, is scheduled for an elective outpatient shoulder scope surgical procedure. The director of the surgery center calls you for advice. What would you tell him/her to do with regard to this patient and specifically about airway management?
Dr. Foley: Although there is no documentation available for the patient’s airway management, it sounds like a possible difficult airway. I would advise the director of the surgery center to have the patient be evaluated, if he or she has not already done so. If the patient is a difficult intubation, I would recommend having the surgery take place in the main OR where there are more resources and help is available. Most of my patients who are undergoing shoulder surgery are in the beach chair position with limited access to the airway. Patients without a difficult airway are usually intubated with an LM airway and an interscalene block. For this patient who has a difficult airway and whose access to the airway during surgery will be problematic, I would advise securing the airway with an ETT. If the LM airway fails while in the sitting position, you will already be behind with the patient desaturating and a difficult intubation ahead.
Dr. Galgon: This is an interesting question because I have had to consider it in my practice. The answer requires planning and consideration. The default position is to take on the case only if the patient’s safety can reasonably be assured for the benefits of doing the procedure at the center. Some things to consider include the following:
- Is the patient scheduled at a freestanding surgery center, at a surgery center adjacent to or attached to a hospital with ICU services, or at an outpatient surgery center that is part of a tertiary or greater hospital?
- Does the surgery center have any policies in place that dictate an outright decision to move the case to another location?
- Can the prior anesthesia records be obtained?
- Does the location offer immediate help from a colleague if needed, or does the center operate on a thin budget for help?
- Is the center appropriately equipped with devices familiar to me to manage a difficult airway in a manner I would prefer, including having equipment to manage a possible can’t intubate, can’t oxygenate (CICO) situation?
I am going to assume the following answers to the above questions:
- The patient is scheduled at a freestanding surgery center.
- The center does not have a policy in place that dictates an outright decision to move the case to another location.
- The prior anesthetic records are not obtainable.
- The center’s staffing provides backup personnel resources, if needed.
- The center is equipped with familiar devices to manage a difficult airway in a manner in which I would prefer, including equipment for a CICO situation.
In this situation, I would tell the director of the surgery center that the patient can be scheduled for the procedure, assuming he/she does not have any other comorbidities that would preclude care at the surgery center, but I would like to be able to meet the patient and examine him/her, preferably in an anesthesia pre-op clinic in advance, and anesthesia staffing on the day of the scheduled procedure must be adequate to ensure immediate help. If the patient’s exam is reassuring and the preparatory steps noted above can be established, then I believe there is a reasonable chance I can manage the patient safely for the planned procedure in the setting described. However, I would not fault any provider who feels uncomfortable with this situation and directs the case to be performed at another location.
Dr. Law: I am assuming that the patient previously had a difficult and traumatic laryngoscopy and intubation, and with concern about iatrogenic upper airway edema, the patient was ventilated overnight. The advice sought now may relate to whether the shoulder case should be done in an ambulatory surgery center (ASC) at all, or may simply address how airway management should proceed on this occasion, regardless of location.
With respect to the former question, regardless of whether the surgical procedure is planned under regional or general anesthesia, in my opinion the case should be done in a hospital with a full array of difficult airway equipment and easy access to expert assistance. This may or may not describe the ASC in question. Regardless of location, given the history, the patient needs a careful preoperative assessment, including physical examination of the airway and review of previous records. This will dictate how airway management should proceed. Without more details, it is hard to say how I would proceed, but given the misery encountered previously, I suspect I would have a pretty low threshold for performing awake tracheal intubation up front.
Dr. Mir: Shoulder arthroscopy is an elective procedure and will require a general anesthetic to facilitate this. In view of the history, I would recommend looking for old notes or information from the patient’s previous anesthetic chart, if possible. If this is not deemed possible, I would recommend a thorough airway assessment and a plan to secure the airway with suitable adjuncts at hand. It would be safer if the airway were secured using an awake tracheal intubation technique. This procedure should not be performed in a day surgery or outpatient setting in view of the previous airway complications.
Dr. Perin: This is a case with the best predictor of a difficult airway, which is a history of a difficult airway! In a typical day, this is a case that you can manage with a peripheral nerve block and an SAD in place. Specifically in this case, I would do it under general anesthesia. I would evaluate the patient and look for predictors of difficulty. If any were present, I would prefer to do an awake endoscopic intubation for the procedure. If “can’t ventilate” predictors are not present, I would do VL as my first choice, and if there is any difficulty I would do an asleep fiber-optic intubation (FOI). After an uneventful intubation, the patient can be discharged from the hospital on the same day. A backup technique would be to place an intubating laryngeal mask and do an FOI through an SAD.
Dr. Straker: As this is an elective procedure, I would try to locate the patient’s records from the previous hospital. In addition, I would have otorhinolaryngology do a preoperative endoscopic examination to visualize the vocal cords from above. Based on the patient’s body habitus, airway exam, and any information that I might be able to locate from the previous records, I would then decide whether to proceed with intubation awake or asleep.
Dr. Urdaneta: There are unique issues with ASCs: First, not all practices are the same. Some facilities are office-based, so the preoperative area, operative area, and recovery beds are similar; some are attached or physically close to a main hospital; while others are freestanding surgical facilities that require transportation to a hospital in case of an unplanned admission or if a complication occurs. Equipment availability, including equipment for rescue options, the availability of ancillary support personnel, and the emphasis on rapid turnover and discharge of patients, are some of the relevant variables that are unique to ASC settings.9
I am of the opinion that equipment availability to handle an expected or unexpected difficult airway should not be a major issue to decide whether to proceed. Every facility should have adequate equipment, including rescue devices, and the personnel capable of handling expected and unexpected airway difficulties. As a provider and after evaluating the patient, I would follow the ASA airway guidelines. The most conservative approach would be not to do the planned procedure in the ASC setting, and do it instead in a facility that affords the possibility of ICU admission in case problems arise. History of a prior difficult intubation is one of the main factors for anticipating airway management problems.
The awake approach is obviously an attractive method by which to instrument the airway of a known or suspected difficult intubation patient, but issues at extubation will remain. The use of an SAD with or without a regional anesthetic block is another option, but not having access to the airway during a shoulder procedure is a valid concern. Regardless of the technique chosen, backup plans and procedures should always be ready and handy.
Dr. Valencia Orgaz: One of the most important predictors of a difficult airway is a history of a difficult airway. My recommendation would be to either perform an awake intubation or, if the surgical team considers it appropriate, sedate the patient and keep him/her under spontaneous ventilation. My best approach would be to use the flexible endoscope. Having said this, plan B would be to use awake VL, and plan C would be to use an awake SAD as a conduit for a flexible endoscopic approach.
Regardless, I would add that airway management is not over once the patient gets intubated and will take precautions for extubation, and maybe even extubate over an AEC.
Dr. Wong: Unplanned ICU admission, ventilation, and sore throat may or may not be the result of a difficult intubation. I would do a careful anatomic and physiologic airway evaluation. I would recommend having this case done in a hospital setting with personnel who are familiar with difficult airway management, and with appropriate equipment.
6.A patient is scheduled for laparoscopic tubal ligation 48 hours after uncomplicated delivery. No other risks factors are involved. Would you electively do the case with the aid of an SAD?
Dr. Foley: My preference would be to not use an SAD for a laparoscopic procedure but rather to secure the airway with an ETT. That said, if I did use an SAD, it would be a second-generation device. Before the start of surgery, I would make sure the SAD was properly placed with the bubble test. I would also make sure I have a seal with an oropharyngeal leak pressure greater than 25 cm H2O or greater than 8 cm H2O above PAP under normal ventilation, as the PAP increases after pneumoperitoneum by 2 to 7 cm H2O. Also, the maximum minute ventilation should be sufficient to remove carbon dioxide following carbon dioxide insufflation and absorption.
Dr. Galgon: No. I am uncomfortable using an SAD for laparoscopic procedures, including tubal ligations.
Dr. Law: I would intubate this patient. My understanding is that studies indicate gastric emptying has returned to normal within a day post-delivery,10 so that absent significant symptomatic reflux, this by itself might not contraindicate use of an SAD for this case. That said, SAD use in a head-down patient with pneumoperitoneum is not my favorite thing. I acknowledge that second-generation SADs might allow better laryngeal sealing than earlier versions, permitting ventilation without “pop-off” at higher airway pressures. However, my preference is for use of tracheal intubation for laparoscopic procedures, even if they are potentially brief cases. With tracheal intubation, I am more assured that I will be able to ventilate in the face of higher airway pressures, and yes, in the recently delivered parturient, I am reassured by having a more protected airway.
Dr. Mir: In the immediate postpartum period, the likelihood of reflux and aspiration risk is unclear and has been debated. In my practice, I would avoid the use of an SAD in the immediate postpartum period due to the following:
- Mechanical effect of a gravid uterus is not present, but the size of the uterus may not be back to normal in the immediate postpartum period. Therefore, the risk for regurgitation and aspiration under an anesthetic may be higher.
- The lower esophageal sphincter tone, which is reduced in pregnancy, may still be incompetent, and the increased risk for aspiration may still be present.
- The possible use of opioids during labor or postpartum for analgesia may contribute to an increased risk for regurgitation.
- Increased intraabdominal pressure, during a laparoscopic procedure, may mimic a full-term pregnancy and therefore present an increased risk for regurgitation and aspiration.
- Immediate postpartum patients are likely to have a high body mass index, so an ETT may be a better option.
In those parturients who have had active reflux during pregnancy, the symptoms may not have fully reversed within 48 hours of delivery. So I would exercise caution and intubate this patient.
Dr. Perin: The direct answer is no. I prefer to separate the answer into two parts:
- The physiology of a pregnant patient after delivery has not changed dramatically in the last 48 hours. Pregnant patients are eight times more difficult to intubate than the general population, and if the patient is morbidly obese, the incidence of a failed airway increases by more than 30%. Gastric contents can be elevated. Besides that, the uterus is still big and can be a problem by compressing the stomach and diaphragm. I would start the case with an ETT in place using a video laryngoscope as a first choice.
- The second aspect is laparoscopic surgery with an SAD. I am not comfortable routinely with this technique because the Trendelenburg position and pressure of the abdominal contents normally can make ventilation very challenging. As discussed, if the patient has gastroesophageal reflux disease, asthma, or chronic obstructive pulmonary disease, the risks of the use of an SAD outweigh the benefits from the technique.
Dr. Straker: Being somewhat of a traditionalist, I would secure the airway with an ETT. The patient still has a “pregnant airway,” is still considered “full stomach,” and is undergoing a laparoscopic procedure. All factors, together, would lead me to intubate the patient.
Dr. Urdaneta: Although there are more reports and even meta-analyses appearing in the literature describing the use of SADs, especially second generation, for laparoscopic procedures, this is still a controversial subject and one that needs more scrutiny and evidence, and not just anecdotal case reports. It may be possible that in the future, SADs will become a primary airway option for minor laparoscopic procedures. But for now, I do not agree with the elective use of an SAD for laparoscopic procedures, unless it is for a rescue airway option. I admit this is subject to change; ask me this same question a decade from now, and my position might have evolved.
The issues with increased ventilation pressures, ventilation requirements due to capnoperitoneum, changes in patient position (i.e., Trendelenburg), and risk for aspiration all nullify the advantages of the use of SADs compared with tracheal tubes—such as ease of placement, less requirement for use of neuromuscular blockers, and lower incidence rates of morbidity, coughing and bucking, and sore throat. I would prefer to intubate with a rapid sequence induction technique and use a cuffed tracheal tube for this laparoscopic procedure.
Dr. Valencia Orgaz: Yes, I would do the case with an SAD. An SAD will help me in case of possible gastric regurgitation, it affords higher sealing pressures, and serves as a great conduit for endoscopic intubation as a plan B in case my original plan fails; but it would have to be a second-generation SAD.
After checking correct positioning of the SAD (including capnography and pressure waves, negative bubble test), I would check the sealing pressure of the device and discuss intraabdominal pressure and patient positioning with the surgical team.
Dr. Wong: The textbook answer is, aspiration risk normalizes 48 hours postpartum. However, in certain patients with conditions such as diabetes or morbid obesity, aspiration risk may still be higher than average. I tend to be conservative and will perform a rapid sequence intubation with an ETT.
7.Does your institution have a dedicated airway cart for the OR and ICUs, the ED, and other locations? Are all of the carts/trolleys organized in the same fashion?
Dr. Foley: We have an airway cart in the main and ASC ORs, as well as in labor and delivery and the ICU and EDs. Each cart is set up similarly to the others.
Dr. Galgon: The short answer to this question is yes. This has been a practice at our institution for over 15 years. Prestaged equipment and standardization ensure equipment availability throughout the institution and equipment location familiarity within the cart. It is important to have a system in place to ensure that the equipment is consistently checked for operability, checked for expiration outdating, and restocked in a timely manner as needed.
Dr. Law: Funny you should ask. Should they be organized in a similar fashion? Absolutely yes, especially if anesthesia personnel are regularly called upon to manage difficult airways in locations outside the OR. Are they organized in a similar fashion at our place? Not so much.
One of our senior residents and an airway fellow recently did a formal audit and presented their findings in an abstract at the 2018 SAM meeting. To quote the abstract, in the 10 carts they audited, “an extensive and diverse accumulation of equipment was found stored in a haphazard manner without standardization.”11 This is poor, and we plan to fix it. Fixing it will not be easy, and as always, it will require good communication between specialties and, I daresay, still more of those lovely end-of-day meetings!
Dr. Mir: Our institution has a difficult airway trolley for the theater area. This is standardized across all areas where anesthetic is given (e.g., the ICU, the ED, interventional radiology, the endoscopy suite and the day surgery unit). They are all organized in the same manner, with four drawers marked A, B, C and D, in accordance with the DAS algorithm for difficult airway management.
Dr. Perin: At my institution, we have the same airway trolley for the OR, ICU and ED. Also, every drawer has the same devices, and all staff are trained in the SimLab with the same trolley. One thing that I think is simple and very good to help decision making is to have plan A, B and C stickers outside each respective drawer.
Dr. Straker: We have dedicated airway carts for the OR. They consist of multiple families of airway devices, local anesthetics, and a monitor for visualization of the airway. The ED and ICU have their own equipment, although they are the same brand of devices that are used by the anesthesiology department. Throughout the hospital, if there is an urgent or emergent airway, the difficult airway response team (DART) can be activated. Although there is not a specific cart on all floors of the hospital, the DART team arrives with a duffel bag of portable airway equipment, which ranges from airway catheters to FFB and VL.
Dr. Urdaneta: I consider this to be an area that deserves more attention. The number and composition of emergency airway equipment should be individualized based on physical needs and requirements. We have two airway carts/trolleys for the OR and a portable bag with rescue airway equipment (both devices and drugs) for out-of-the-OR emergency management.
Having said this, we do not stock or organize the carts/trolleys for outer peripheral areas, such as the ED and ICUs, and recognize this as a latent factor of patient safety. I agree it would be best to organize the emergency airway equipment/drugs uniformly for various settings throughout the whole hospital. For our portable bag, we always try to emphasize the importance of proper and uncluttered stocking and storage of all pieces of equipment and drugs, but often our efforts are thwarted, and inevitably either the bag does not get restocked after some piece gets used, or more commonly we have redundant supplies that are not needed. Stocking of the portable bag requires constant monitoring and education.
Dr. Valencia Orgaz: Yes, we have difficult intubation carts for the OR area. Our ORs are located on two different floors, and we have one cart for each of them. We do not have a specific cart for out-of-the-OR airway management, but we are working to make them available soon.
Our airway carts are stocked and labeled as per the DAS recommendations, with the same equipment and following a logical pathway:
- Plan A: intubation
- Plan B: ventilation
- Plan C: rescue ventilation
- Plan D: surgical airway
We also have a portable backpack with basic tools: a laryngoscope, bougies, video laryngoscopes, SADs and an AEC for out-of-the-OR airway emergencies.
Dr. Wong: We have an identical dedicated difficult airway cart in the OR and medical–surgical ICU.
References
- Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2013;118(2):251-270.
- Cook TM, Woodall N, Harper J, et al. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth. 2011;106(5):632-642.
- Peterson GN, Domino KB, Caplan RA, et al. Management of the difficult airway: a closed claims analysis. Anesthesiology. 2005;103(1):33-39.
- Chrimes N. The Vortex: a universal “high-acuity implementation tool” for emergency airway management. Br J Anaesth. 2016;117(suppl 1):i20-i27.
- Alhomary M, Ramadan E, Curran E, et al. Videolaryngoscopy vs. fibreoptic bronchoscopy for awake tracheal intubation: a systematic review and meta-analysis. Anaesthesia. 2018;73(9):1151-1161.
- Royal College of Anaesthetists. RCoA-DAS airway leads. www.rcoa.ac.uk/?clinical-standards-quality/?rcoa-das-airway-leads. Accessed May 1, 2019.
- Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg. 2007;105(5):1357-1362.
- Roten FM, Steffen R, Kleine-Brueggeney M, et al. Dislocation rates of postoperative airway exchange catheters—a prospective case series of 200 patients. BMC Anesthesiol. 2019;19(1):52.
- Gormley G, Mannion S. Airway management in ambulatory anesthesia. Curr Anesthesiol Rep. 2014;4:342-351.
- Whitehead EM, Smith M, Dean Y, et al. An evaluation of gastric emptying times in pregnancy and the puerperium. Anaesthesia. 1993;48(1):53-57.
- Garza M, Phipps S, Milne A, et al. An audit of adult difficult airway carts within three academic teaching centres in Halifax, Nova Scotia. Presented at the Society for Airway Management Annual Meeting; September 13-16, 2018; Chicago, IL. Abstract.











Leave a Reply
You must be logged in to post a comment.