Dear Rapid Response:
Transporting patients is a high-risk process, accounting for up to 5% of pediatric anesthesia adverse events.1 Studies have identified respiratory and airway adverse events as some of the most common complications, along with the role of transport equipment in reducing risk.2 The role of equipment in safe patient transport highlights the importance of human factors engineering in the design of medical devices utilized by health care providers. Human factors engineering considers the capabilities and limitations of humans and addresses the interface design of equipment to promote safe, reliable, and efficient use in various situations.3,4 Using a human factors perspective, we would like to describe the design of a pressure valve found on the SunMed Ventlab HS4000 Series Hyperinflation System (Figure 1, Figure 2, Ref. HS4011, Ventlab, LLC; Grand Rapids, MI). This product temporarily replaced our existing Jackson-Rees transport circuits due to supply shortages at our institution.
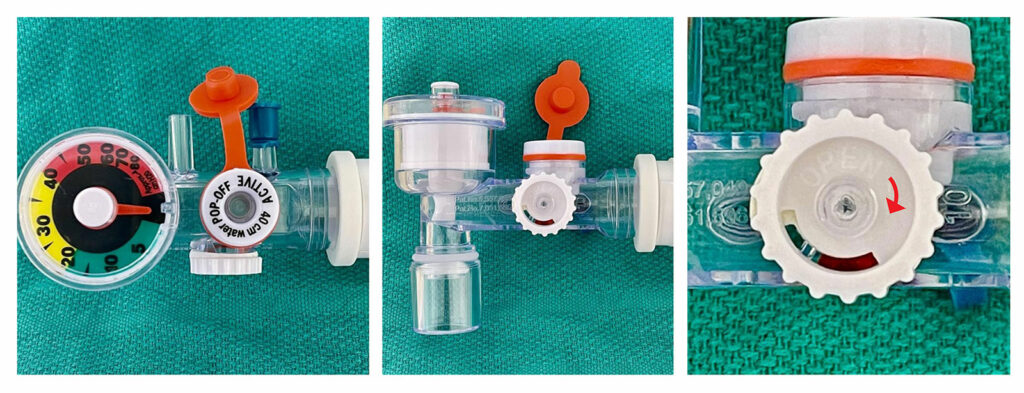
Figure 1: Multiple views of the SunMed Ventlab Hyperinflation System (Ref. HS4011, Ventlab, LLC, Grand Rapids, MI) with attention to the Adjustable Pressure Valve. Note that the white text on white plastic is difficult to read. The clockwise arrow is labeled “Open”—which is the opposite of what is typically expected (where clockwise rotation usually leads to closure of a valve).
Figure 2: Full packaging of the SunMed Ventlab Hyperinflation System (Ref. HS4011, Ventlab, LLC; Grand Rapids, MI).
The SunMed Ventlab Hyperinflation System includes a color-coded pressure manometer and a pressure adjustment valve. The manufacturer describes the valve as a “stay-put dial” to set a static pressure. However, our institution’s health care providers found the interface for adjusting the dial counterintuitive: increasing pressure requires counter-clockwise rotation, and decreasing pressure requires clockwise rotation. Our perioperative staff found this design to be atypical compared to all other hyperinflation devices used in our hospital. The familiar adage “righty-tighty, lefty-loosey” that helps guide people to rotate right, or clockwise, to tighten an apparatus and left, or counter-clockwise, to loosen does not apply in this device’s design. Furthermore, the dial is made of white plastic with a label indicating the direction of turn that is difficult to read due to lack of color contrast (Figure 1, right panel). The counterintuitive design of the dial confused providers during patient transport, which had the potential for delayed care, specifically in a critical scenario where effective positive pressure ventilation is required. Realizing this design difference, rapid education was conducted with perioperative care providers using this hyperinflation system.
For anesthesia professionals especially, a comparison can be drawn between the pressure dial on the hyperinflation system and the adjustable pressure-limiting (APL) valve on anesthesia machines. The International Organization for Standardization (ISO) has standards that apply to the design of all APL valves on anesthesia machines. In regulatory standard ISO 80601-2-13:2011, exhaust valves, which include APL valves, should have their pressure adjusted such that clockwise rotation closes the valve and increases circuit pressure, and counter-clockwise rotation opens the valve and decreases the pressure.5 In other words, “righty-tighty, lefty-loosey.” The APL valve is used day-in and day-out by anesthesia professionals. Thus, when encountering another flow-dependent oxygen delivery device with a valve, anesthesia professionals are likely to attempt to turn a valve clockwise in order to close it to increase pressure delivered to the patient based on their familiarity with this standard.
Given ongoing supply chain challenges, providers often face substitute devices that may not be equivalent to the device they are accustomed to using. Furthermore, supply chain managers should work closely with clinicians to ensure that design differences that may have patient safety implications are addressed when making substitutions. In the case of the SunMed Ventlab Hyperinflation System, the dial design is opposite to the commonly available design of the Mapleson circuits that were routinely used at our institution. This counterintuitive design is a potential patient safety issue, and clinicians should be aware of this limitation if faced with these devices. In this time of disrupted supply chains, there is often little lead time to maintain desired inventory, but as much as possible, supply chain managers should confirm that a product is clinically acceptable before making a substitution. Furthermore, appropriate in-service education may help mitigate potential issues from arising from use of unfamiliar substitute devices.
Thank you for your concern and attention to this matter.
James Xie, MD
Jonathan Barnett, MD
References
- Haydar B, Baetzel A, Stewart M, et al. Complications associated with the anesthesia transport of pediatric patients: an analysis of the wake up safe database. Anesth Analg. 2020:131:245–254. PMID: 31569160.
- Haydar B, Baetzel A, Elliott A, et al. Adverse events during intrahospital transport of critically ill children: a systematic review. Anesth Analg. 2020: 131;1135-1145 PMID: 32925334.
- Weinger MB, Slagle J. Human factors research in anesthesia patient safety: techniques to elucidate factors affecting clinical task performance and decision making. J Am Med Inform Assoc. 2002:9;S58-S63. PMCID: PMC419421
- Weinger MB, Gaba DM. Human factors engineering in patient safety. Anesthesiology. 2019:120;801-806. PMID: 24481419.
- Medical electrical equipment Part 2–13: Particular requirements for basic safety and essential performance of an anesthetic workstation. Geneva: ISO, 2011: ISO 80601-2-13:2011. https://www.iso.org/obp/ui#iso:std:iso:80601:-2-13:ed-1:v1:en.
Response:
We appreciate the opportunity to respond to the article on the Ventlab HS4000 series hyperinflation system with integrated manometer and pop-off, one of the most widely used hyperinflation systems in the market today.
When introducing customers to new products, SunMed believes education is key. It is important for clinicians to be educated on products prior to use as devices may have different features. Different, however, does not mean counterintuitive when performance features are understood.
SunMed provides:
- Comprehensive Instructions for Use
- Training and education
- In-service support for conversions across our breadth of products
Education includes how to control and interpret the pressure relief valve. The valve within the hyperinflation system does not contain an APL (adjustable pressure-limiting) valve as the report makes comparison to, and therefore, is not intended to function similarly. Instead, the Ventlab Hyperinflation System device functions like most frequently used hyperinflation systems on the market and comes with a pressure relief valve that rotates forward, closing the valve and restricting flow (increasing pressure), or inversely, rotates backwards, opening the valve (reducing pressure). The pressure relief valve located on the side of the device was designed by a clinician with consideration for human factors and ease of use. The valve allows for one-handed adjustment with the thumb during use, while continuously monitoring the pressure on the integrated manometer and/or the patient. Additionally, the valve comes with a visual aid to leverage the benefits of visual indication through a red indicator window which provides added ease-of-use when identifying the position of the valve (full red = fully closed, no red = fully open) prior to and during utilization.
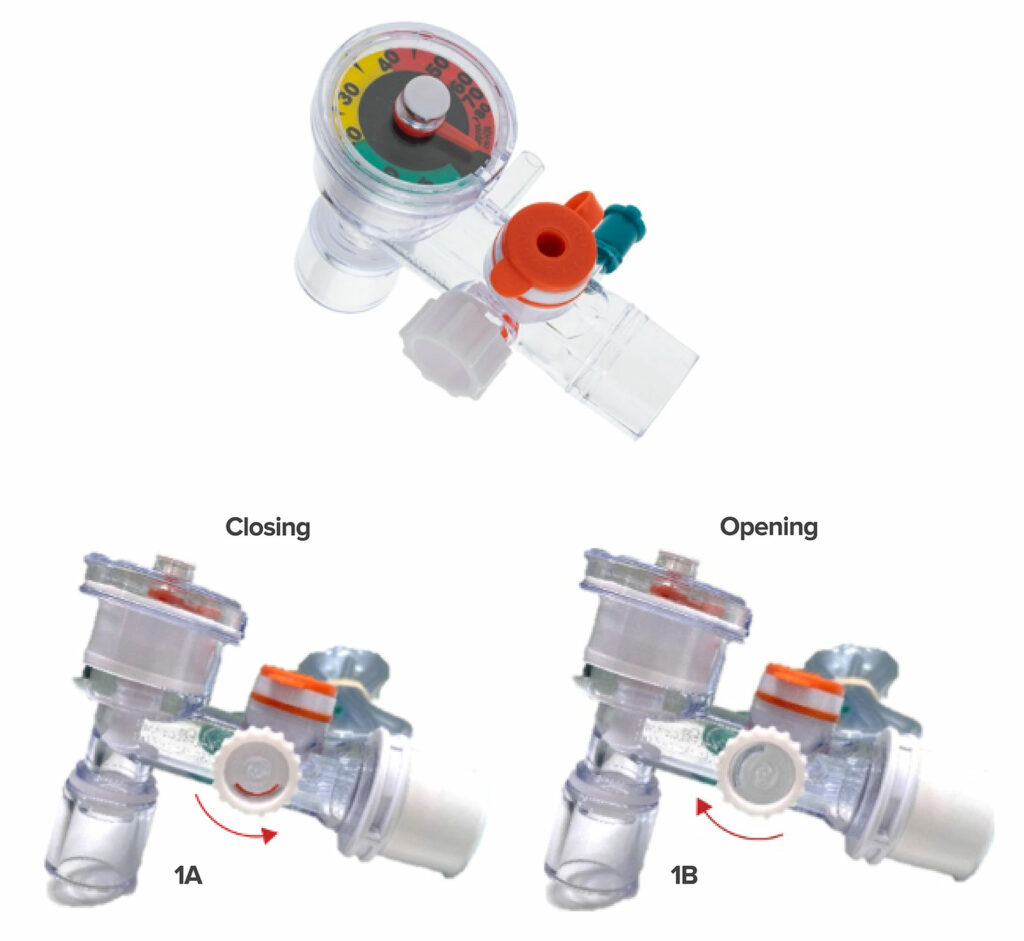
Figure 1: The Ventlab Hyperinflation System device comes with a pressure relief valve that rotates forward (1A) closing the valve and also rotates backward, thereby opening the valve (1B) and reducing pressure.
SunMed thanks the authors for sharing this report and for the feedback which is welcomed as part of our culture for continuous improvement. SunMed also appreciates the opportunity to discuss the clinical design benefits of the Ventlab Hyperinflation System and the critical importance of product training.
Sincerely,
Gary Banks
SunMed
Senior Director of Marketing Respiratory,
2710 Northridge Dr. NW, Suite A I Grand Rapids, MI 49544 I USA
Jessica Hoke
SunMed
Sr. Vice President RAQA & EHS
2710 Northridge Dr. NW, Suite A I Grand Rapids, MI 49544 I USA


Leave a Reply
You must be logged in to post a comment.