Case reports in anesthesia
Sunday afternoon the friendly GI doc boarded a case for an urgent ERCP in a 31 year old female with choledokolithiasis. She had elevated liver enzymes and he was concerned that she might develop ascending cholingitis. The patient had hypothyroidism and was morbidly obese with a weight of apporoximately 320 lbs and was 5’3″. We proceeded to the OR after 2 mg versed, 100 mcg fentanyl and 4 mg decadron were given as a premed. In the OR after monitors were applied, pre oxygenation in the reverse T – burg position with HOB up at 30 degrees was accomplished. Induction with 150 mg of propofol and succinylcholine (100mg) was used. Intubation was easy grade I view. However, She immediately desaturated (less than 60 seconds). Manual ventilation was begun to expand lung units, and sevoflurane was begun and saturations slowly climbed to the high 90’s. Within 4 to 5 minutes, the patient began moving slightly. I immediately went to increase the sevoflurane, but noticed at this point that the vaporizer was completely empty. Unfortunately, the machine only allowed one vaporizer at a time to be inserted into the port. Therefore, I had to remove the sevo cartridge and insert the cartridge with desflurane. The patient was now starting to move and cough, causing her to desaturate again. I overpressurized the desflurane to quickly deepen the anesthetic to prevent further coughing and bucking on the ETT. The patient, again desaturated significantly (down into the 60’s). With careful and aggressive manual ventilation to expand all alveoli units, her sats slowly started to climb and the desflurane was able to be delivered. In an attempt to quickly deepen the anesthesia, I had turned the desflurane up to 10%. After getting her sats back up into the 90’s, I switched again to machine ventilation with pressure control of 29 and PEEP of 9 mmHg. However, within a short period, her sats began to fall again. I decreased the desflurane to 6% and once again took over manual ventilation. I perceived that indeed her compliance was very poor. I quickly verified once again that her ETT was not in too deep (i.e. right main stemmed), and that there were no kinks in the ETT. I also gave 40 mg of rocuronium to ensure that she wasn’t fighting the ventilator and rule out any chest wall contribution to poor compliance. With careful high pressure ventilation I was able to achieve large tidal volumes (not achievable with the anesthesia machine despite high pressure settings in pressure control ventilation). Her saturations again trailed down into the 60’s. At this time the ERCP was underway, and the endo team was particularly involved with the procedure. The anesthesia tech had left for the day (I’m not sure why). I suspected bronchospasm at this point (previously thinking I was dealing with atelectisis with shunt due to morbid obesity). Therefore, I asked for help in filling the vaporizer. During this time I was forced to put her back on the ventilator with saturations in the 80’s. I quickly filled the sevo vaporizer, and replaced the desflurane with sevoflurane and ran it at 4% with high FGF (i.e. 5 l/m). After about five minutes, her compliance began to improve, her saturations slowly improved for the remainder of the case. Now that I could rely on mechanical ventilation, I was free to grab some albuterol and hook up a make shift nebulizer treatment via the ETT and ventilator which required some creative hookups to achieve success. The case lasted about 50 min. At the end of the case, compliance was pretty near normal levels with normal saturation’s. However, the patient was still weak from the very large dose of rocuronium given to ensure improved compliance. Given that she only had one twitch and the Concern for airway patency, I administered 200 mg suggamadex. I followed this dose with a subsequent dose of 100 mg to achieve full reversal in about 3 minutes. The patient went to PACU extubated on face mask O2 and saturations at 100%.
This case highlights the interplay of obesity, asthma (bronchospasm/reactive airway disease) and desflurane.
Obesity has reached epidemic proportions in the US. Super obese patients require surgery for a variety of problems and can present unique problems for the anesthesia team. In this case, the patient reported no significant past medical history with the exception of hypothyroidism. However, severe hypoxemia was encountered almost immediately after induction, which was exacerbated by significant bronchospasm likely brought on by high (overpressurization) concentrations of desflurane.
Pathophysiology of the obese state
Excess adipose tissue is associated with the production of various proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1-β (IL-1β), and interleukin-6 (IL-6). TNF-α plays a critical role in the inflammatory response of the immune system as well as in the apoptosis of adipose cells, lipid metabolism, hepatic lipogenesis, and the induction of oxidative stress. Increased levels of TNF-α promote a response via the release of IL-6, another proinflammatory molecule, and the reduction of levels of anti-inflammatory cytokines such as adiponectin. TNF-α also increases the interaction of electrons with oxygen, generating superoxide anions. TNF-α levels are elevated in obese individuals and decrease with weight loss [1]. Furthermore, Adipose tissue is a source of several bioactive adipokines, including leptin, adiponectin, visfatin, resistin, apelin, and type I plasminogen activation inhibitor (PAI-I). These adipokines are directly associated with physiological and pathological processes involving oxidative stress [2]. Rasslan et al. [3] observed that adipose tissue is an endocrine and paracrine organ that produces many cytokines and bioactive mediators, resulting in a pro-inflammatory state that may be associated with pulmonary hypoplasia, atopy, hyper reactive bronchi, and increased risk of asthma in obese individuals. Leptin is one hormone elevated in the obese state, likely related to leptin resistance and consequent overproduction. leptin crosses the blood brain barrier and serves as an afferent signal, originating from the adipose tissue, engaging distinct hypothalamic effector pathways to suppress appetite and augment energy expenditure. Therefore, leptin is an important negative feedback loop in controlling appetite to prevent obesity. Leptin resistance has been shown to enhance parasympathetic tone which increases airway reactivity and obesity associated asthma [8]. In addition, hyperleptinemia was proposed to be a crucial factor leading to respiratory failure in leptin-resistant obese subjects [9]. Meta-analyses have further implicated a positive relationship between serum leptin and the risk of asthma. Of note, the link with obesity and airway hyper responsiveness is much stronger in females than males. Furthermore, it has been shown that Leptin expression is inhibited by testosterone, whereas it is increased by ovarian sex steroids and therefore, leptin is higher in females than age and body mass index (BMI) matched males. Of note, leptin also plays a role in central respiratory function. Injection of leptin into the nucleus of the tractuss solitarius increases minute ventilation in addition to enhanced bioelectrical activity of the respiratory muscles. This seems to indicate that in obesity with leptin resistance, patients will be at much higher risk for OSA partially related to the inability of the brain to react to high leptin levels. In obese patients, hyperleptinemia is associated with a reduction in respiratory drive and hypercapnic response, irrespective of anthropometric measurements while circulating leptin is a predictor for the presence of hypercapnia. Furthermore, as discussed above, high leptin levels result in the production of a number of cytokines that induce inflammation. Administration of leptin to wild-type mice enhances O3-induced airway inflammation (in other words, airways are hyper reactive in mice who are given exogenous leptin). In summary, while the evidence relating leptin levels to lung and respiratory pathology is mixed, there seems to be a general preponderance of studies linking obesity, leptin resistance (i.e. elevated leptin levels) and hyper responsive airways.
Therefore, the obese state creates altered hormonal levels from the non obese state that have a pro inflammatory effect and promote hyper responsive airways.
In addtion to the above described endocrine pathology of obesity, Mafort et al. evaluated 30 patients and showed that the primary change in lung volume was reduced end respiratory volume. In the morbidly obese, FVC and FEV1 were both reduced. However, obesity has been associated with a higher incidence, prevalence and severity of asthma and with altered pulmonary function, poor treatment response, and high morbidity. The increased incidence is correlated with BMI in that there is a 35% increase in the risk of asthma with each 3 unit increase in BMI. Further derangements of lung function in obese individuals have been identified. Radial traction of lung parenchymal attachments around the airways is attenuated at low lung volumes contributing to airway collapse. Breathing at low lung volume is a hallmark of respiration in morbid obesity. When an obese patient goes from standing to supine, there is no further decrease in FRC and expiratory reserve volume (contrary to what is seen in lean people). But, Airway resistance increases with decreased expiratory flow. As intrinsic (or auto) PEEP develops, work of breathing (WOB) increases and the supine obese patient develops a mixed respiratory pathology of restrictive and obstructive etiology. In the supine obese patient oxygenation is particularly affected by breathing at low lung volumes where expiratory reserve volume approaches residual volume. A decreased expiratory capacity seems to be the primary cause of decreased ventilation/perfusion ratios causing significant and rapid hypoxemia after induction of anesthesia. Therefore, pre oxygenation with pressure support and PEEP in a head up (at least 25 degrees), can increase safe apnea time. After intubation, recruitment maneuvers with titrated PEEP can optimize oxygenation. Obese patients are far more susceptible to ventilator induced lung injury (VILI) due to the potential for high driving pressures to ensure adequate ventilation. The dual concepts of static stress and dynamic strain on the lungs come into play both adding to the risk of VILI. By titrating PEEP appropriately, both of these can be mitigated to a degree. Large PEEP values (at least 12 cmH20) may be needed, however. As the degree of end expiratory lung pressures increase, the right ventricle will be exposed to increased afterload. However, at very low lung volumes, right afterload is also increased due to collapsed vasculature. Therefore, the obese patient will need to be well hydrated to counter act the potential negative CV effects of increased levels of PEEP.
It should be noted, that the majority of the increase in degree of airway resistance in patients receiving Desflurane were in those who were smokers. Even still, non smoking patients who received Desflurane saw no decrease in airway resistance like those who received Sevoflurane. In 2008, a study [7] in pediatric patients comparing Desflurane to Sevoflurane in normal patients and in those with airway senstivities was able to show that Sevoflurane decreased airway resistance slightly in both groups, while Desflurane increased airway resistance. (graphic)
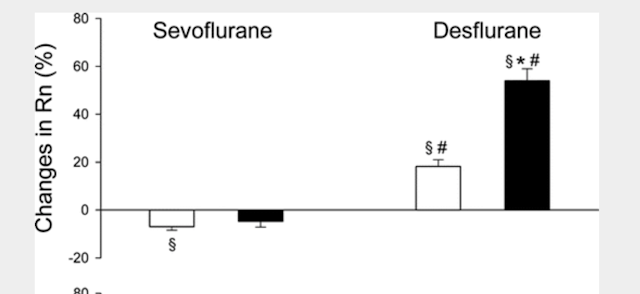 |
| light bar is normal patient, and the dark bar are patients with airway sensitivity. |
More recently, a case series in the PICU was able to show that in six children with life threatening asthma exacerbations, Sevoflurane successfully relieved bronchospasm and all children made improvement except one who was later diagnosed with ARDS and required NO. Consequently, while desflurane has a superior recovery profile vs Sevoflurane in obese patients due to its very low solubility co efficient in blood and fat, in some patients, the potential for increased airway resistance may present problems. In my patient, who was super obese, a severe bronchospasm was elicited after introduction of Desflurane with overpressurization. Sevoflurane was able to break this bronchospasm successfully. The patient was required to return for a lap chole three days after her ERCP. The team used sevoflurane. I arrived towards the end of the case to relieve the provider who had started the case. On the hand over report I was told that she had saturated in the high 80’s for much of the case, but this improved to mid 90’s after the evacuation of the pneumoperitoneum. She was maintained on Sevoflurane, and upon emergence and transfer to the PACU she did well although she required oxygen. On POD #2, she required an additional ERCP due to more stones found in the duct. After intubation, recruitment breaths were used, and she was placed on 9 cmH20 of PEEP with pressure control ventilation of 30 cm H20. Sevoflurance at 2.4% was used. She did not experience any evidence of bronchospasm, and saturations were maintained at 98% on Fio2 of 0.85. She was extubated at the end of the case and went to PACU without incident.
References
1. Möller K, Ostermann AI, Rund K, Thoms S, Blume C, Stahl F, et al. Acids. 2016;106:39–49.
2. Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, et al. Int J Mol Sci. 2014;16:378–400.
3. Rasslan Z, Stirbulov R, Lima CA, Saad JR. Lung function and obesity. Rev Bras Clínica Médica. 2009;7:36–9.
4. Grassi L, Kacmarek R, Berra L. Anesthesiology. 2020;132:1246-56.
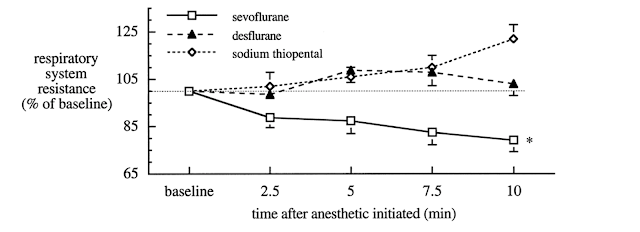
5. Rooke GA, Choi J-H, Bishop MJ: . anesthesiology 1997; 86:1294–9
6. Goff MJ, et al. Anesthesiology 2000, Vol.93, 404-408
7. von Ungern-Sternberg BS, Saudan S, Petek F. et al. Anesthesiology 2008, Vol.108, 216-224
8. Arteaga-Solis, E. et al. Cell Metab. 2013;17, 35–48
9. Phipps, P. R., Starritt, E., Caterson, I. & Grunstein, R. R. Thorax 57, 75–76 (2002).


Leave a Reply
You must be logged in to post a comment.