DEAR RAPID RESPONSE:
In 2013, my colleagues and I reported a case of hypoventilation to the APSF Newsletter due to a massive leak from a defective Drägersorb disposable absorbent canister (CLIC®) (Drägerwerk AG & Co. KGaA, Lübeck, Germany), which was replaced during surgery while using the Fabius GS premium anesthesia workstation (Drägerwerk AG & Co., Lübeck, Germany).1 Since our report appeared in the APSF Newsletter, similar cases have been reported on other Dräger anesthesia machines such as Perseus A5002 and Primus.3 Despite these reports, there have been no specific actions to prevent future occurrences, so the risk of hypoventilation after replacing a CO2 absorber during surgery has continued. Furthermore, this risk is not unique to Dräger anesthesia machines although the implications of a leaky canister are different depending upon the machine design. In this report, our prior experience is summarized and the impact of a leaky canister on ventilation is described for different machine designs. To inform anesthesia professionals about this risk, a “WARNING” should be added to the Instructions for Use (IFU) for all anesthesia machines that provide the option to change the absorbent canister during a procedure.
When the Fabius GS machines were introduced to our department, it was the first time we could replace the absorbent canister during a procedure. This practice was adopted to more completely utilize the absorbent. For the case we reported in the previous APSF Newsletter, the canister was changed during the procedure without apparent problems. At the end of the procedure, when switching to manual ventilation, the breathing bag collapsed and could not be inflated, despite maximizing the fresh gas flow (FGF) or repeatedly pressing the oxygen flush valve. The patient’s endotracheal tube was disconnected from the anesthesia machine and ventilation continued with a Jackson-Rees circuit and an external oxygen tank. The patient restored spontaneous breathing and was extubated uneventfully. Investigation revealed a large hole in the absorbent canister as the reason for the inability to create pressure in the circuit. The unique design of the Fabius machine, which incorporates a fresh gas decoupling valve, allowed for adequate mechanical ventilation, but complete ventilation failure in manual mode.
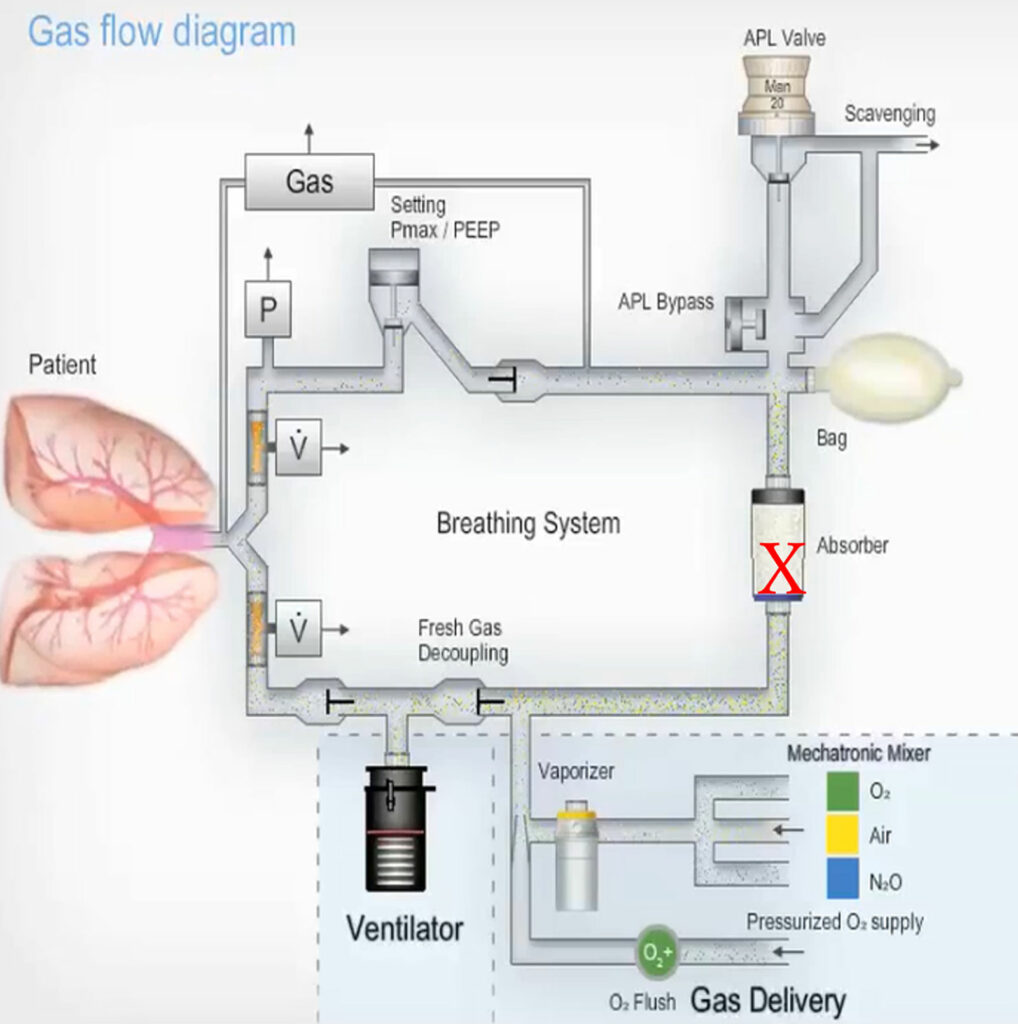
The current anesthesia workstations with piston ventilators manufactured by Drägerwerk AG & Co. have a unique design that uses a fresh gas decoupling (FGD) valve in the breathing system to prevent fresh gas from entering the circuit during inspiration. The CLIC adaptor makes it possible to replace an absorbent canister during surgery. The FGD valve is located between the piston-type ventilator and the fresh gas inlet, and a CO2 absorber is located between the fresh gas inlet and the breathing bag (Figure 1). The FGD valve ensures inspiratory pressure is maintained during mechanical ventilation even if a leaky absorbent canister is correctly attached to the CLIC adaptor. During the expiratory phase of mechanical ventilation, as the piston retracts, ambient air can be drawn from the defect in the absorber into the anesthesia circuit. The gas concentrations in the circuit are altered by the entrained ambient air but, depending upon the size of the leak, the concentration changes may not be readily apparent. With manual ventilation, however, positive pressure is created by the reservoir bag and a defect in the canister can then make ventilation impossible with a collapsed breathing bag and potential injury to the patient.
Figure 1: Schematic of Dräger Piston-type Breathing Circuit (Primus/Apollo, Fabius Models) with leak site indicated with an X. During mechanical inspiration, the FGD valve will close preventing gas from leaking via the canister. During exhalation, when the piston draws gas from the reservoir bag, ambient air can be entrained into the circuit through the canister leak. When manual ventilation is attempted, positive pressure from the bag will cause gas to leave through the canister leak making manual ventilation difficult, if not impossible, depending upon the size of the leak. (Courtesy Dräger Medical.)
APL = Adjustable Pressure Limiting; FGD = Fresh Gas Decoupling.
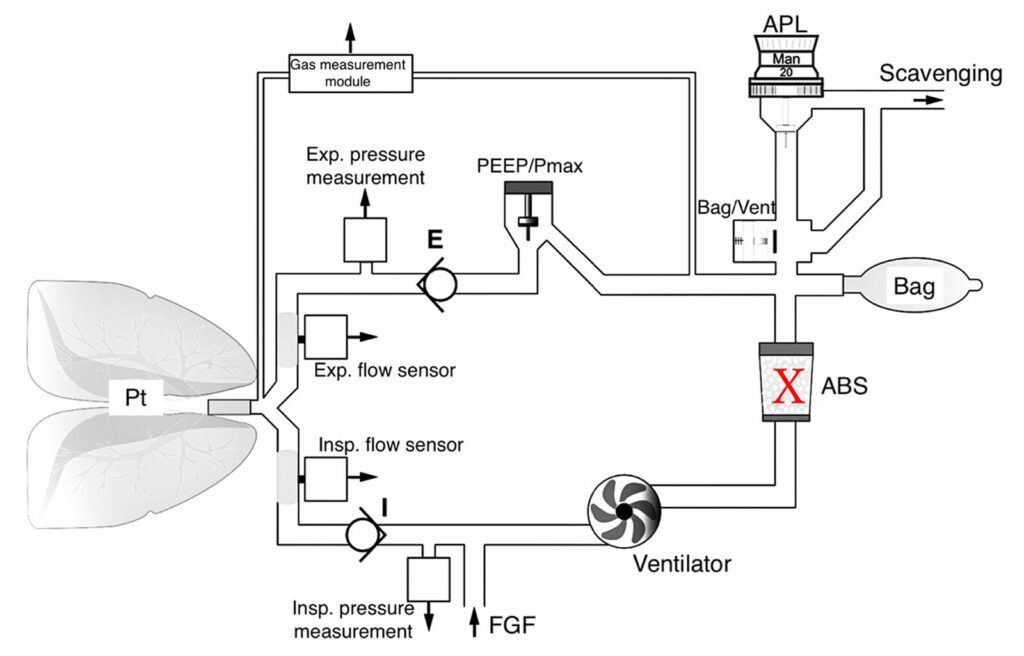
The anesthesia workstations with turbine-type ventilators (Dräger Perseus and Zeus) do not have a FGD valve, but the breathing bag fills with fresh gas and functions as a reservoir for the ventilator (Figure 2).4 During mechanical ventilation, inspired gas is taken from the FGF and the reservoir bag. When there is a leak in the absorbent canister,2 during mechanical ventilation, tidal volume is not altered, but the gas concentrations in the circuit are altered as ambient air can enter the circuit through the canister. The impact on gas concentrations depends upon the size of the leak and the total FGF with a greater impact for larger leaks and lower FGF. Since the breathing circuit has unidirectional flow, fresh gas should continue to fill the reservoir bag during mechanical exhalation. Manual ventilation may be difficult or impossible depending upon the size of the leak.
Figure 2: Schematic of Dräger Turbine Type Breathing Circuit (Perseus) with leak site indicated with an X. Mechanical inspiration will continue if there is a canister leak although ambient air can be entrained altering the concentration of gases in the circuit. During exhalation, exhaled and fresh gas will continue to fill the bag. Manual ventilation may be difficult or impossible depending upon the size of the leak. (Courtesy Dräger Medical.)
ABS = Absorbent canister; FGF = Fresh gas flow; APL = Adjustable pressure limiting.
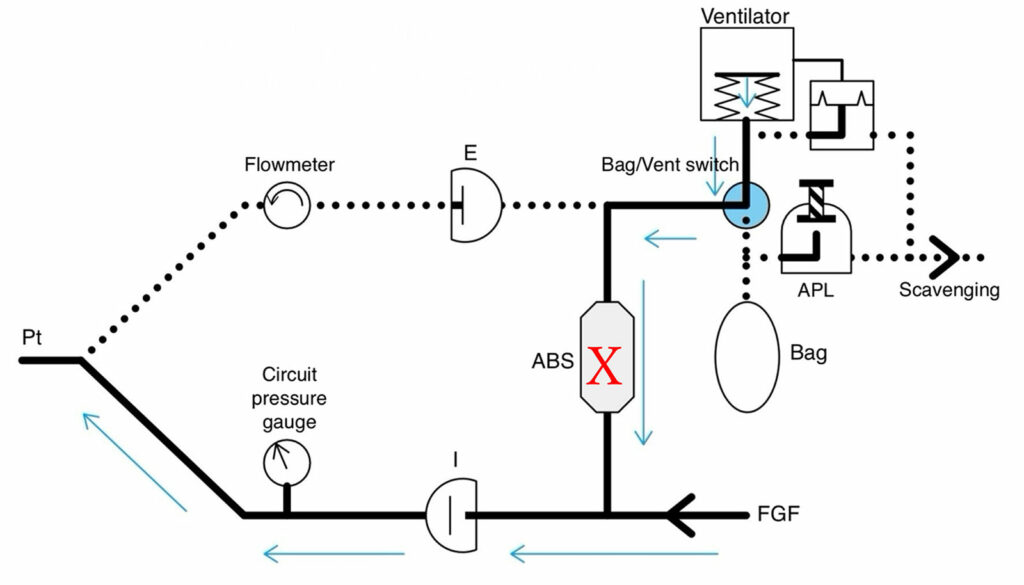
Dräger is not the only manufacturer to offer absorbent replacement during surgery. GE Healthcare (Madison, WI), Mindray North America (Mahwah, NJ) and Getinge USA (Mahwah, NJ) all offer the same feature, and the machine design will determine the impact on the breathing circuit of placing a canister with an undetected leak. There is a report of leakage occurring in a GE anesthesia machine after replacing a damaged disposable canister.5 GE and Mindray anesthesia machines have an ascending bellows ventilator and no FGD valve. During mechanical ventilation, the reservoir bag is excluded from the circuit. If there is a leak in the canister, the bellows will collapse during inspiration and make mechanical ventilation impossible (Figure 3).6 During manual ventilation, the bellows is excluded, but the reservoir bag will collapse making ventilation impossible. The size of the leak will determine how quickly either the bellows or reservoir bag collapse, but if either occurs there is a leak until proven otherwise and the canister may be the source if it was recently changed.
Figure 3: Schematic of Bellows Type Breathing Circuit (GE and some Mindray Models) with leak site indicated with an X. Both mechanical and manual ventilation are impacted similarly if there is a canister leak since they are both in the same place in the circuit and selected by the bag/vent switch. In all cases, positive pressure ventilation can be difficult or impossible depending upon the size of the leak. The leak will be indicated by either collapse of the bellows or collapse of the reservoir bag. (Figure created by Dr. Kuruma.)
ABS = Absorbent canister; FGF = Fresh gas flow; APL = Adjustable pressure limiting.
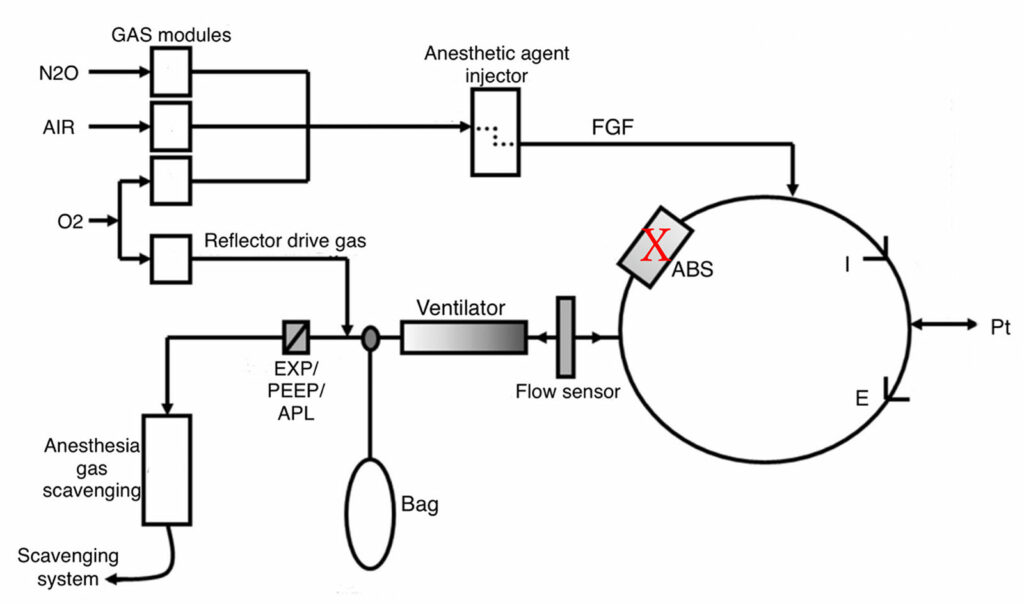
Another type of anesthesia machine circuit design is the volume reflector, which is included in Getinge anesthesia machines and the Mindray A9. While there are no published reports of a leaky canister with the volume reflector design, the consequences can be understood by inspecting the circuit design (Figure 4).7 In this circuit, a ventilator and a breathing bag are located upstream of the absorber. Like the bellows design, during mechanical ventilation, the reservoir bag is excluded from the circuit, and during manual ventilation, the ventilator is excluded. The volume reflector ventilator provides a continuous supply of 100% oxygen as the drive gas. Under normal conditions, the drive gas pushes gas to the patient, but does not enter the patient circuit. In the event of a canister leak, the drive gas may provide some ventilation to the patient depending upon the size of the leak, but will dilute the anesthetic in the circuit and change the oxygen concentration. Manual ventilation may not be possible if there is a large leak, and the bag will collapse.
Figure 4: Schematic of volume reflector type breathing circuit (Getinge, Mindray A9) with leak site indicated with an X. During mechanical ventilation, the volume reflector will provide a continuous source of 100% oxygen. If the leak is not too large, some inspired tidal volume may be delivered, but the oxygen can dilute the anesthetic in the circuit and change the oxygen concentration. The bag is excluded during mechanical ventilation. Manual ventilation may be difficult or impossible depending upon the size of the leak. (Courtesy Getinge.)
ABS = Absorbent canister; FGF = Fresh gas flow; APL = Adjustable pressure limiting.
Current practices intended to reduce the environmental footprint when using a circle anesthesia system include reducing FGF and using the CO2 absorbent to completion. Achieving the absorbent goal requires waiting to change the absorbent until inspired CO2 is present,8 which is the rationale for allowing an intraprocedure exchange. While there is benefit to this feature, our experience and other reports underscore the risks of an undetected canister leak. While a leaking canister will be detected by the preoperative leak test, it is only apparent by ventilation failure and/or changes in gas concentrations when replaced during a procedure.
Therefore, my colleagues and I are reluctant to follow the practice of replacing the absorber during surgery due to the risk of personnel failing to recognize the problem and respond in a timely fashion, potentially leading to patient harm. Instead, we continue to rely upon the color change of the absorbent to determine when to replace the canister. Typically, the canister is replaced before the start of anesthesia and a leak test is done after the replacement. Leaks in the canister will therefore be detected before patient care. When a long procedure is planned, we replace the canister beforehand in order to reduce the possibility of replacement during surgery. Unfortunately, we have not been able to take full advantage of the CLIC adapter on the Dräger anesthesia machine.
The problem of inability to detect a leaky canister until it results in difficulty ventilating is inherent to the design of modern anesthesia machines. Piston ventilators from Dräger with a FGD valve and turbine ventilators without a FGD valve will continue mechanical ventilation and the problem may not be apparent until switching to manual ventilation. Other ventilator designs (bellows and volume reflector) should demonstrate a failure to ventilate fairly soon after canister replacement. Manufacturers who provide the ability to change absorbent during a procedure should inform users about the risk of an undetected canister leak and the problems likely to result depending upon the circuit design. We suggest that all manufacturers add an appropriate “WARNING” to the IFU of their anesthesia workstations. In the case of Dräger piston ventilators, we propose the following example:
WARNING
Replacement of a CLIC disposable CO2 absorbent canister during a procedure has the attendant risk of impossible manual ventilation if the replacement has an undetected leak. Due to the FGD valve, mechanical ventilation will not be altered significantly if there is a canister leak. Visual inspection of the canister is essential to detect any defect of the disposable canister before replacement. After intraprocedure canister replacement, tidal volume and inspiratory pressure as well as gas concentrations in the circuit should be carefully monitored for any changes. A manual resuscitation device, auxiliary oxygen supply and intravenous anesthetics should always be readily available to prevent patient injury in the event of an anesthesia machine failure.
REFERENCES
- Kuruma Y, Kita Y, Fujii S. Exchanging a CLIC absorber in the middle of the surgery. APSF Newsletter. 2013;27:64–65. https://www.apsf.org/article/exchanging-a-clic-absorber-in-the-middle-of-the-surgery/ Accessed August 13, 2024.
- Watanabe H, Moriyama K, Tokumine J, et al. Massive leak in carbon dioxide absorber of Perseus A500 did not inhibit mechanical ventilation but manual bag ventilation: a case report with experimental reproduction. A A Pract. 2021;15:e01425. PMID: 33740784
- Rey A, Malezieux O, Potie A. The fresh gas flow decoupling valve and the potential for leaks in the anaesthetic circle breathing system. Anaesth Rep. 2021;9,e12141. PMID: 34881364
- Instructions for use. Perseus A500 SW 1.1n ( https://www.draeger.com/Content/Documents/Content/IfU_Perseus_A500_SW_1.1n_EN_9054101.pdf. Accessed August 9, 2024)
- Sano H, Suzuki T, Kaneda Y. Gas leak due to a damaged GE disposable multi absorber canister used with an EZchange module following its reinstallation during anesthesia. Can J Anesth. 2015;62:96–97. PMID: 25326266
- Kaminoh Y. LiSA. 2015;22:984–990. (in Japanese)
- Lucangelo U, Ajcevic M, Accardo A, et al. FLOW-I ventilator performance in the presence of a circle system leak. J Clin Monit Comput. 2017;31:273–280. PMID: 27062381
- Feldman JM, Hendrickx J, Kennedy RR. Carbon dioxide absorption during inhalation anesthesia: a modern practice. Anesth Analg. 2021;132:993–1002. PMID: 32947290
Editor’s Note: Intraprocedure Replacement of CO2 Absorbent Canisters
by Jeffrey Feldman, MD, MSE
The circle anesthesia system is specifically designed to reduce inhalation agent waste and greenhouse gas pollution by allowing the anesthesia professional to reduce fresh gas flow causing rebreathing of exhaled anesthetics. Carbon dioxide absorption is required to safely and effectively reduce fresh gas flow. Carbon dioxide absorbents also contribute to the waste stream and offset the advantages gained by reducing fresh gas flow although the net benefit favors reducing fresh gas flow.1 Changing absorbent based upon the appearance of indicator alone increases absorbent waste by discarding unused absorbent. To minimize absorbent waste, it is useful to utilize the absorbent until it becomes ineffective, which is indicated when inspired CO2 begins to appear in the capnogram.2 This practice is only practical when using an anesthesia machine designed to allow for absorbent canister replacement without interrupting positive pressure ventilation or anesthetic delivery. The major anesthesia machine manufacturers all provide options that allow for intraprocedure replacement of absorbent canisters.
In this issue of the Newsletter, Yuki Kuruma, MD, revisits her previously published report of manual ventilation failure due to replacing the absorbent canister during a procedure with one that has a leak due to a crack or hole in the housing.3 In the current article, Kuruma emphasizes that the risk of failed ventilation due to replacing a faulty canister intraoperatively has not changed since the original report in 2013. Indeed, all of the existing machine designs have that risk, and Kuruma reviews how the impact of a faulty canister can manifest depending upon the machine design. Furthermore, the manufacturers that offer an option for replacing the absorbent during a procedure have not provided specific warnings about the risks of doing so if the canister has a leak nor any best practice for mitigating the risk.
CO2 absorbent canisters are typically plastic housings containing absorbent material with engineered adapters unique to each anesthesia machine manufacturer. During shipping and stocking, it is quite possible for these canisters to be damaged in a manner that causes a leak when placed in the breathing circuit. The pre-use checkout, whether it is automated or manual, should detect any leaks in the absorbent canister. When the canister is changed intraoperatively, however, it is not practical to perform a leak test since an alternate method of anesthetic delivery and ventilation would be required. As a result, the clinician must rely upon inspection of the canister to identify any potential leaks as well as vigilance once the canister is changed for any untoward impact. The problem is that it can be difficult to identify all sources of leaks by inspection alone.
Depending upon the machine design, placing a leaky canister into the circuit during a procedure will cause changes in gas or anesthetic concentrations and/or failure to ventilate manually, mechanically or both. Furthermore, even if there is no problem with the new canister, when it is first placed, it contains only room air and will alter the concentrations in the circuit as the volume of gas in the canister reaches equilibrium with the rest of the circuit. This change in concentration is especially noticeable when using low fresh gas flow.
Kuruma’s suggestion to provide a warning in the IFU, while desirable, is not likely to prevent problems since the IFU is not reliably read. There are some additional options for practices that could help to identify a leaky canister and mitigate patient risk if a leaky canister is placed intraoperatively.
- Before replacing the canister, inspect the new canister for any signs of damage or cracking. If any are present, select another one from inventory.
- After replacing the canister, reduce fresh gas flow and provide several manual breaths by squeezing the reservoir bag and observing the monitored values for inspiratory pressure and delivered tidal volume. If it is difficult to create the desired pressure or deliver the intended tidal volume, a leak in the canister should be suspected. This procedure should be useful for all anesthesia machine designs since manual ventilation is impacted similarly in all of the machines.
- Increase fresh gas flow for a few minutes after the integrity of the canister is confirmed and monitor the gas concentrations in the circuit to foster the mixing of desired gas concentrations inside the new canister.
While these practices should help to identify a leaky canister and prevent patient harm, there are some additional possibilities for ensuring the canister is intact before intraoperative replacement.
- Perform a leak test on a supply of absorbent canisters using an anesthesia machine and store these tested canisters in a protected box to be available for replacement.
- Develop a device that can be used to pressure test a canister before it is placed into service. Since the intraoperative change adapters are standardized for each manufacturer, the companies are well positioned to design a pressure testing device that could reside in a supply room for testing a replacement before it is used.
The practice of changing absorbent canisters intraoperatively based upon an inspired CO2 threshold is a desirable method to minimize the amount of unused absorbent that is discarded, thereby reducing absorbent waste. The information provided here is not intended to discourage the practice of intraprocedure absorbent replacement, but to ensure that providers are aware of the impact of a canister leak. Guidance is provided to help mitigate the risk to patients. Manufacturers of canisters designed for intraoperative replacement are encouraged to provide an appropriate warning and consider recommending best practices for detecting leaks, or developing methods for testing canisters for leaks before they are placed into service.
REFERENCES
- Feldman JM, Lo C, Hendrickx J. Estimating the impact of carbon dioxide absorbent performance differences on absorbent cost during low-flow anesthesia. Anesth Analg. 2020;130:374–381. PMID: 30925559
- Feldman JM, Hendrickx J, Kennedy RR. Carbon dioxide absorption during inhalation anesthesia: a modern practice. Anesth Analg. 2021;132:993–1002. PMID: 32947290
- Kuruma Y, Kita Y, Fujii S. Exchanging a CLIC absorber in the middle of the surgery. APSF Newsletter. 2013;27:64–65. https://www.apsf.org/article/exchanging-a-clic-absorber-in-the-middle-of-the-surgery/ Accessed August 13, 2024.
Dräger Anesthesia Workstations & Intraoperative CO2 Canister Exchange
by David Karchner, MBA; Hans Ulrich Schuler, MSEE, MBA; and Bjoern Goldbeck, MSEE
DEAR EDITOR,
We would like to thank Yuki Kuruma, MD, for her article in this issue of the APSF Newsletter where she reviews the risk of introducing a leak into the breathing circuit following intraprocedure replacement of the CO2 absorbent canister. We also thank the APSF for the opportunity to respond to Dr. Kuruma’s submission.
Sustainable practices that reduce waste are important. In anesthesia practice, CO2 absorbent canister disposal presents an opportunity to minimize waste. Towards that end, many vendors like Dräger have implemented options that support replacing the CO2 canister during a procedure, enabling users to utilize a larger percentage of each canister’s CO2 absorbent potential instead of replacing the canister at the beginning of the procedure when the CO2 absorbent is not completely utilized.
Each of Dräger’s anesthesia machines provides the opportunity to choose between the traditional “loose fill” CO2 absorbent, which are always refilled when the anesthesia machine is not in use, and the “CLIC” canister, which provides the opportunity to replace the canister during a procedure based upon evidence that the absorbent is almost completely utilized like elevated inspired CO2. The loose fill approach inevitably discards useful absorbent material, whereas the CLIC canister minimizes the waste of useful absorbent. Regardless of the strategy, it is important for clinicians to understand the CO2 absorbent canister is part of the breathing system, and introducing a canister with a leak can negatively impact the ability to ventilate the patient.
As Kuruma’s submission indicated, the option for intraprocedure canister replacement is not unique to Dräger, and there will be different responses from each anesthesia machine design when/if a leak is introduced with a damaged CO2 absorbent canister. While gas concentrations may change with a leak, the inability to ventilate mechanically, manually, or both could also occur. Kuruma observed that it was possible to continue mechanical, but not manual, ventilation when installing a CO2 canister with a leak that cannot be overcome by increasing fresh gas flow, in a machine with piston or turbovent ventilators. This author requests that anesthesia machine manufacturers provide a warning in their Instructions for Use (“IFU”) outlining this risk. In response to the report to APSF in 2013 by Kuruma et. al., various explicit warnings, and additional information have been included in the different IFUs of Dräger anesthesia workstations and into the IFU of the CLIC Absorber itself (Figures 1-3).
Figure 1: Apollo IFU (page 117) outlining the need for “greater attention” when changing the absorber during operation.
Figure 2: Warning in the IFU of the Apollo Anesthesia Workstation (page 118) informing that changing parts of the breathing system, including the CLIC Absorber, may alter the leak or the compliance of the breathing system.
Figure 3: Several warnings are included with the IFU of the CLIC Absorber and CLIC Adapter intended to mitigate the risk of patient injury. Inspecting the CLIC Absorber for damages before use is an important first step. (Instructions for use. CLIC Absorber 800+ / Infinity ID CLIC Absorber 800+ / CLIC Adapter. Dräger Medical. English page 14.)
Similar warnings are presented in the IFUs for the Perseus A500 and Atlan A350.
In addition to these warnings, Dräger anesthesia workstations are equipped with monitoring devices and associated alarms to help identify problems related to intraoperative placement of a leaky absorbent canister. Gas concentration monitoring is essential to safe anesthesia practice and undesired alterations in oxygen, and anesthetic concentrations are easily detected when using appropriately set alarm limits. Breathing circuit pressure and volume alarms are also important to identifying circuit leaks.
We thank Yuki Kuruma, MD, again for bringing the risk of intraprocedure canister exchange to the attention of the anesthesia community and to our attention as a manufacturer. With this information, we as the manufacturer can continuously improve and update our IFU of the relevant medical devices and support users to be better prepared to avoid patient harm.
Intraoperative CO2 Canister Exchange When Using GE HealthCare Anesthesia Systems
by John Beard, MD, and Robert Meyers, BS
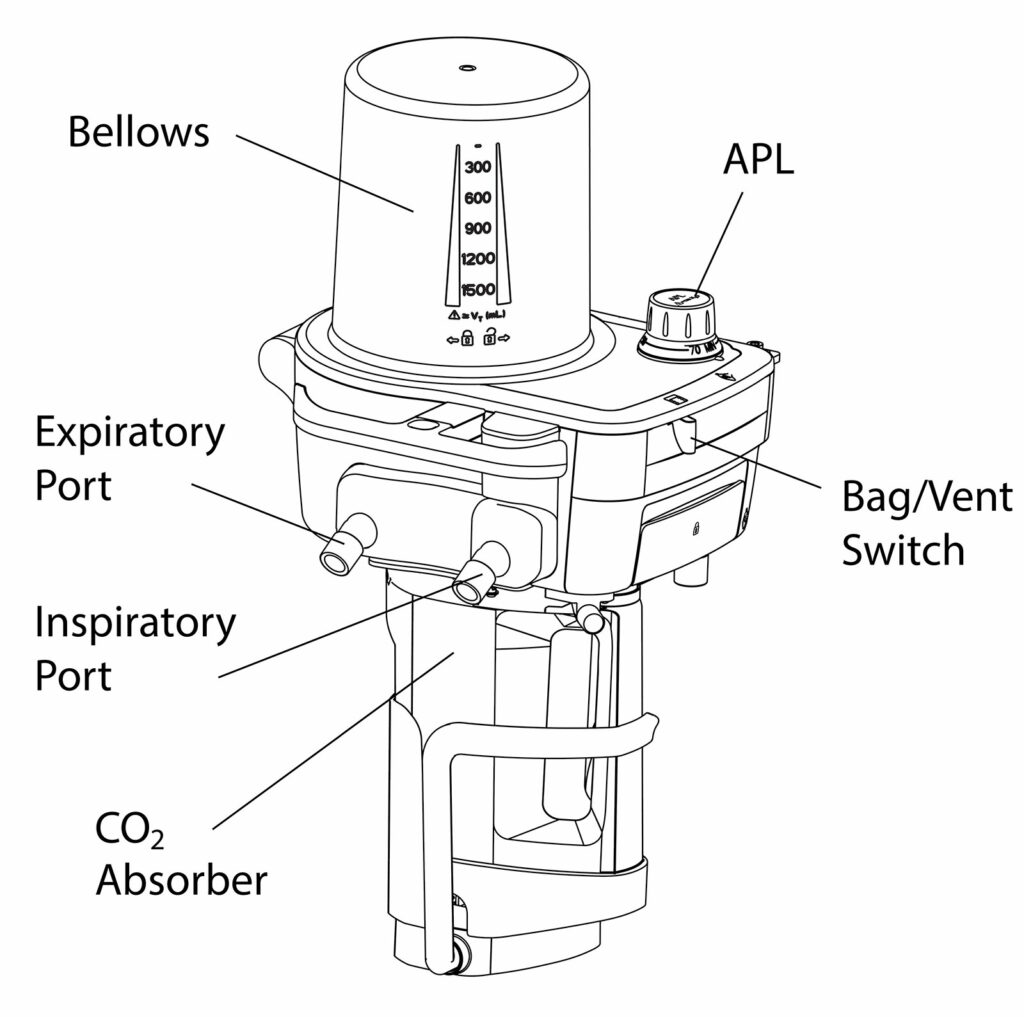
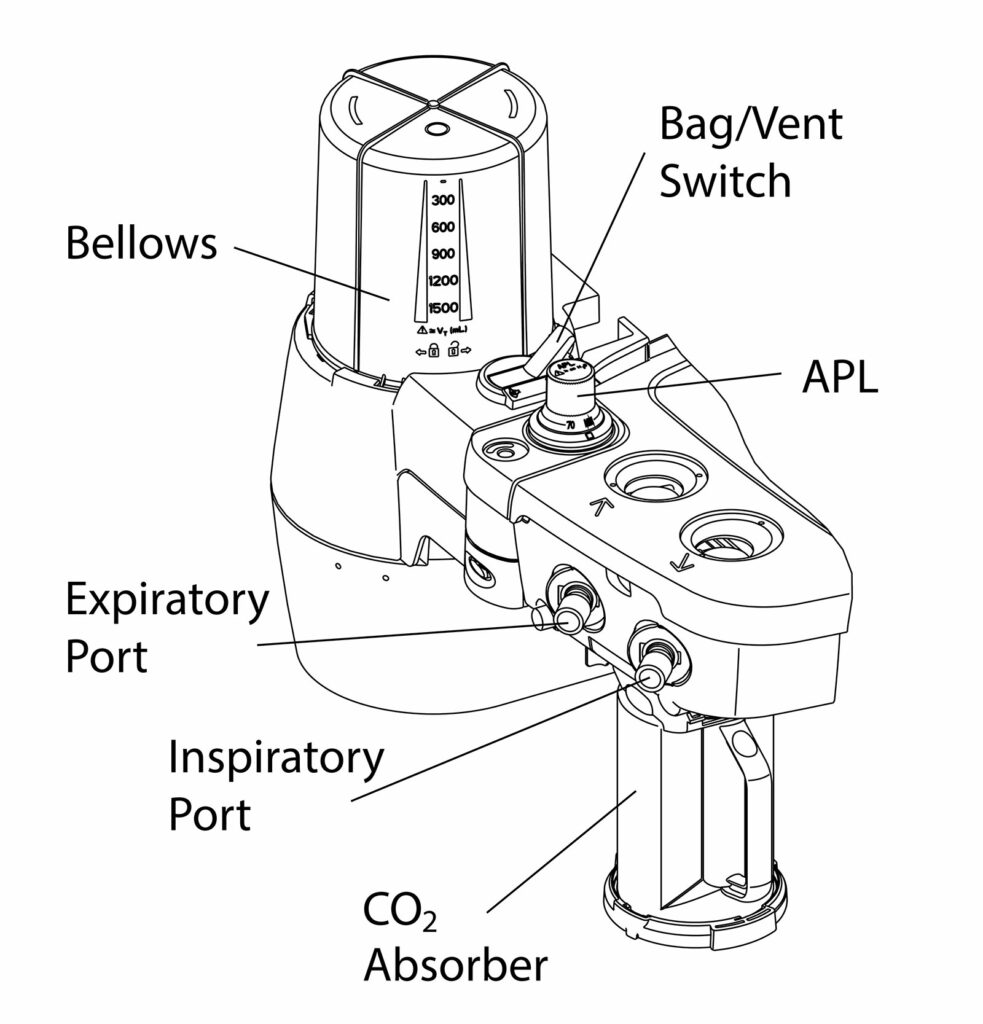
GE HealthCare anesthesia systems can support intraoperative exchange of carbon dioxide (CO2) canisters. GE HealthCare anesthesia systems use a bellows design (conceptual diagram, Kuruma, Figure 3) with either the Compact Breathing System (CBS) or the Advanced Breathing System (ABS) depending on the anesthesia machine family. The CBS (Figure 1) supports intraoperative exchange of a CO2 canister as a standard configuration while the ABS (Figure 2) requires the addition of the optional EZ Change Canister Module.
The CBS is designed with a cam-style lifting mechanism that raises the lower tray (nest) and aligns the absorbent canister with the breathing system ports. The lifting assembly is designed to seal the canister in the breathing system and resist latching if there is any misalignment. When equipped with the optional EZ Change Canister Module, the ABS uses a rotating mechanism to guide the absorbent canister connectors into the mating ports, and is also designed to resist latching if the absorbent canister ports are not aligned. When the absorbent canister is not latched, both systems will display the informational message “CO2 Absorber Out of Circuit” in the waveform area.
Additionally, disposable canisters (AMSORB Plus, Coleraine, Ireland) sold and distributed by GE HealthCare, are pressure tested at the manufacturer prior to shipping to ensure that leakage does not exceed 10 mL/min at 30 cmH2O.
In the rare scenario where an intraoperative exchange of a CO2 canister is associated with a breathing system leak, the bellows-based design of both the ABS and CBS mitigates the impact of the leak during both mechanical and manual ventilation for the following reasons:
- In scenarios where the fresh gas flow is greater than the breathing system leak, there will not be any impact on ventilation or patient gas concentration. The breathing systems in a bellows-based design are positive pressure in operation. This means room air will not be entrained through a canister leak and thus, patient gas will not be diluted by room air.
- In scenarios where the leak is greater than the fresh gas flow, it may still be possible to provide some positive pressure ventilation by either the bag or ventilator. Depending upon the size of the leak, some or all of the intended tidal volume may not reach the patient, and the bag or bellows will eventually collapse. Alarms will be triggered as described below.
- The bellows is a physical barrier between the patient gas and the ventilator drive gas. In the event of a leak in the canister, drive gas will not enter the breathing system and alter the concentration of patient gas.
GE HealthCare anesthesia systems also may help the clinician recognize the leak for the following reasons:
- The bellows and bag provide visual indicators of a leak.
- The bellows is always visible to the clinician. When a leak is greater than the fresh gas flow, the bellows will collapse providing a visual indication to the users.
- When the bag is selected, the bag will collapse when positive pressure ventilation is attempted.
- As outlined in the “Alarms and Troubleshooting” section of the anesthesia machine User’s Reference Manual,3,4 the ABS and CBS systems also provide several alarm messages to help clinicians successfully detect a leak.
- “System Leak?” alarm: This alarm occurs when the drive gas flow from the ventilator is greater than the flow measured by the inspiratory flow sensor (by ~30%) and will help to detect a decrease in delivered tidal volume. The CO2 canister is between the drive gas and the inspiratory flow sensor making this the primary alarm to detect this failure prior to the bellows collapsing.
- “TV not Achieved” alarm: This alarm occurs when the volume measured by the inspiratory flow sensor is less than the set tidal volume by ~10% for six mechanical breaths in a row. This alarm will occur in a volume targeted ventilation mode once the bellows collapses far enough to impact ventilation.
- “Unable to Drive Bellows” alarm: This alarm occurs when the system detects that the ventilator driving pressure does not result in an equivalent increase in airway pressure. Like the “TV not Achieved” alarm, this alarm will occur once the bellows collapses far enough to impact ventilation.
- “TVexp Low” alarm: This alarm occurs when the measured tidal volume is less than the user set alarm level. This alarm will occur once the bellows collapses far enough to impact ventilation.
When a clinician identifies a leak with a CO2 absorbent canister, there are a number of solutions.
- If the leak is not too large, the quickest remedy is to attempt to raise the fresh gas flow above the level of the leak. If that is successful, the bellows (or bag) will reinflate and allow ventilation to continue until the problem can be resolved.
- If the leak is too large to be compensated by increasing fresh gas flow, an alternate method of ventilation (e.g., Mapleson circuit) should be employed and intravenous anesthesia considered.
- Once safe ventilation is assured, the leak in the breathing system can be resolved by exchanging the faulty canister.
In conclusion, GE HealthCare anesthesia systems can support intraoperative exchange of CO2 canisters. In the rare scenario where an intraoperative exchange of the CO2 canister is associated with a breathing system leak, the systems are designed to mitigate the impact of the leak, and also provide visual indicators and alarms to help clinicians recognize and address the leak.
REFERENCES
- GE HealthCare Compact Breathing System Cleaning, Disinfection and Sterilization User’s Reference Manual. Datex-Ohmeda, Inc.; 2014.
- GE HealthCare Advanced Breathing System Cleaning, Disinfection and Sterilization User’s Reference Manual. Datex-Ohmeda, Inc.; 2012.
- GE HealthCare Aisys CS2 User’s Reference Manual. Datex-Ohmeda, Inc.; 2013.
- GE HealthCare Carestation 750/750c User’s Reference Manual. Datex-Ohmeda, Inc.; 2017.