Anesthesiology News
Cleveland Clinic Lerner College of Medicine of Case Western Reserve University
Director of Clinical Research
Department of General Anesthesiology and Outcomes Research
Anesthesia Institute, Cleveland Clinic
Cleveland, Ohio
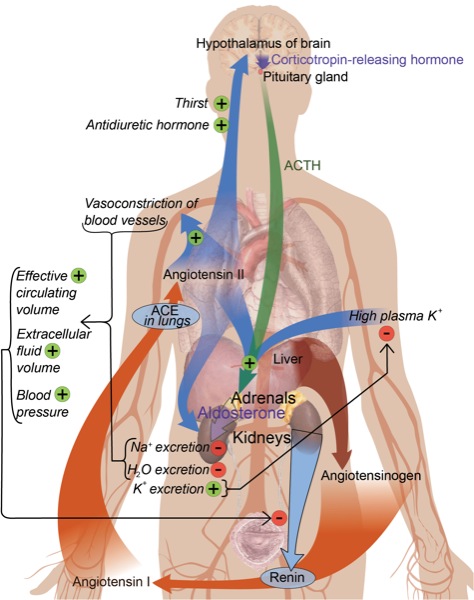
Since the discovery of renin in 1898, the renin–angiotensin system (RAS) is considered a crucial signaling system for adjusting sodium homoeostasis, body fluid volume, and maintaining arterial blood pressure, with physiologic effects mediated via the interaction of angiotensin (Ang) II with Ang type 1 (AT1) receptors. With the discovery of new RAS functions such as vasodilation, neuroprotection, and cognition, these new RAS concepts are now referred to as the “alternate” RAS.1,2 This review focuses on new developments in understanding the functions of the RAS.
Biosynthesis of the RAS and Alternate RAS
The main component of the classic RAS is Ang II, which is generated by a 2-step proteolytic process from the precursor angiotensinogen. In the first step of this process, renin mediates the proteolytic conversion of angiotensinogen generated by the liver to the decapeptide Ang I. In the second step, Ang I is converted to the octapeptide Ang II by angiotensin-converting enzyme (ACE).1 Ang II can be synthesized intracellularly from angiotensinogen and renin. Of note, high glucose concentrations can stimulate both smooth muscle cell chymase and cathepsin D to form intracellular Ang II from Ang I and angiotensinogen.3 Ang II also may be generated from Ang I by chymase under some pathologic conditions, such as the presence of vascular damage associated with atherosclerosis. Chymase is a chymotrypsin-like serine protease that is stored in inactive complexes with heparin proteoglycan in secretory granules of mast cells. Ang II can be produced via the chymase pathway from Ang (1-12) in the human heart. The alternate chymase-dependent pathway predominates in the diabetic kidney. Thus, the use of ACE inhibitors alone without AT1 receptor blockers (ARBs) does not completely prevent albuminuria in diabetic patients because Ang II can still be produced via the renal alternate chymase pathway.4,5
Ang (1-7), the major component of the alternate pathway, can be generated from Ang I by neprilysin, thimet oligopeptidase, or prolyl endopeptidase through the removal of the last 3 amino acids of the Ang I precursor molecule. The ACE homologue, ACE2, is central to Ang (1-7) production. This mono-carboxypeptidase removes the amino acid leucine from the C-terminus of Ang I to form the biologically active peptide Ang (1-9), which is subsequently cleaved to generate Ang (1-7) through ACE and neprilysin.
The second method by which Ang (1-7) is produced is via Ang II. This occurs by the removal of C-terminal phenylalanine from Ang II by ACE2 to form the heptapeptide. Of note, ACE2 has 400 times greater affinity for Ang II than Ang I. Unlike ACE, ACE2 does not metabolize bradykinin; however, it hydrolyzes the pro-inflammatory kinin, Des-Arg9-BK. Therefore, ACE2 has vasodilation and anti-inflammatory effects.1
Ang III (angiotensin 2-8 heptapeptide) is produced from Ang II by aminopeptidase A, whereas Ang IV (angiotensin 3-8 hexapeptide) is produced from Ang II by aminopeptidase M.
RAS and Alternate RAS Receptors
AT1 Receptors
Ang II mediates its effects by binding to G protein–coupled receptors: the AT1 and Ang type 2 (AT2) receptors. Activation of the AT1 receptors stimulates phospholipase C, causing hydrolysis of phosphatidylinositol 4,5-bisphosphate to form inositol 1,4,5-trisphosphate and diacylglycerol. Inositol 1,4,5-trisphosphate activation increases myoplasmic calcium concentrations, and diacylglycerol signaling causes protein C activation and vascular smooth muscle (VSM) contraction.6 In addition, activation of the AT1 receptor induces inhibition of 3’,5’-cyclic adenosine monophosphate signaling, thus enhancing VSM contraction. Ang II activates the NADH/NADPH system (ie, nicotinamide adenine dinucleotide [NAD] hydrogen/NAD phosphate hydrogen), resulting in elevated levels of reactive oxygen species (ROS), including hydrogen peroxide (H2O2), hydroxyl radical, and superoxide anion (O2–).
Elevated ROS reduce nitric oxide (NO) availability, thus enhancing the vasoconstrictor effect of AT1 receptor activation.1 In addition, the interaction between NO and O2– generates peroxynitrite, a cytotoxic anion that inhibits mitochondrial electron transport and destroys DNA and cellular proteins, thereby leading to oxidative stress damage.7 ROS impair mitochondrial function and adenosine triphosphate (ATP) production, with a subsequent increased release of ROS by the mitochondria themselves, initiating a vicious cycle of increasing ROS.
The production of ROS by Ang II augments the inflammatory response by activating a nuclear factor-kappaB (NF-kappaB) transcription factor, enhancing cytokine transcript production.8,9 Ang II can also induce apoptosis through the ROS-mediated inhibition of the antiapoptotic protein Bcl-2.10 Ang II induces VSM hypertrophy by increasing intracellular H2O2.9 AT1 receptor–associated protein (ATRAP) has 3 transmembrane domains that interact with the C-terminal domain of the AT1 receptor. Activation of ATRAP leads to internalization of the AT1 receptor and its subsequent downregulation.
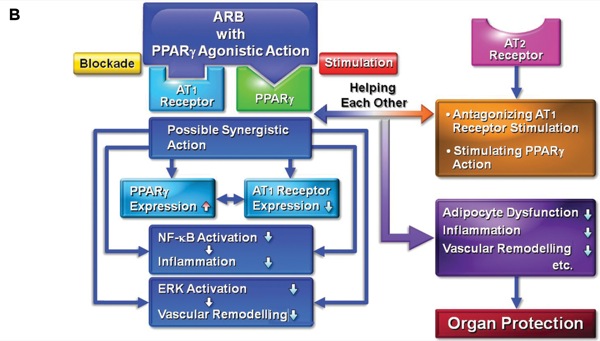
The activation of peroxisome proliferator–activated receptor gamma (PPAR-gamma) inhibits AT1receptor action and stimulates the AT2 receptor.11 Of note, ARBs such as telmisartan and irbesartan have a partial PPAR-gamma agonistic effect. Correspondingly, telmisartan has neuroprotective effects against ischemic brain damage and cognitive dysfunction in a mouse model of Alzheimer’s disease via the synergistic effects of AT1 receptor blockade and PPAR-gamma stimulation (Figure 1).12,13
Figure 1.
AT2 Receptor Functions and Associated Proteins
The AT2 receptor is mainly stimulated by Ang (1-7), and it produces effects counterbalancing those from AT1 receptor stimulation. That is, activation of the AT2 receptor results in vasodilation, NO release, and inhibition of proliferation and growth. Upregulation of the AT2 receptor may exert neuroprotection in the penumbra of ischemic brain tissue, which may explain the observation in the LIFE (Losartan Intervention For Endpoint Reduction In Hypertension) study that administration of the ARB losartan offered superior stroke protection compared with atenolol.14The interaction of the C-terminal tail of AT2 receptor with the mitochondrial tumor suppressor gene-1 results in reduction of O2– production, the expression of pro-inflammatory cytokines, neointimal formation, and atherosclerosis.15 Moreover, the interaction of the AT2 receptor with its associated proteins could result in attenuated tumor growth, vascularization, and metastasis in different models of cancer.
Mas Receptor
Mas is a proto-oncogene that was identified by Santos et al.16 The Mas receptor hetero-oligomerizes with the AT1 receptor and thereby inhibits the actions of Ang II. Therefore, the interaction of Ang (1-7) with the Mas receptor mediates antiproliferative and antiarrhythmic effects, leads to vasodilation via bradykinin and NO release, and stimulates renal sodium excretion. Recently, angioprotectin has been shown to bind with higher affinity than Ang (1-7) at the Mas receptor.17
Prorenin and Renin Receptors
Renin is an aspartyl protease that consists of 2 homologous lobes, with the cleft between the lobes containing an active site consisting of 2 catalytic aspartic residues. Prorenin has an amino-terminal prosegment that folds over the cleft between these 2 lobes, preventing the activation of angiotensinogen. Prorenin is activated in the renal juxtaglomerular cells by enzymes such as proconvertase and cathepsin. In the presence of low pH or cold, prorenin can be activated by unfolding from the interlobe cleft by nonproteolytic activation.18 Both prorenin and renin stimulate the single receptor that is known as (P)RR. Binding of (P)RR to its ligands results in unfolding prorenin, rendering it capable of contributing to local Ang II production. Moreover, renin and prorenin binding causes a rapid phosphorylation of the (P)RR on serine and tyrosine residues, triggering mitogen-activated protein kinase pathways. Consequently, stimulation of (P)RR can have the same harmful effects as AT1 receptor stimulation by Ang II.19,20
Renin–Angiotensin Receptors As Determinants of Life Span
ROS produced by the interaction of the AT1 receptor and Ang II enhance DNA damage through a telomere-independent pathway via the induction of stress-induced premature senescence and a telomere-dependent mechanism via accelerated attrition of telomeres.21 Of note, telomeres play a very important role in protecting the ends of chromosomes from deterioration or from fusion with neighboring chromosomes.
ROS enhances cerebral endothelial dysfunction that occurs with age, thereby old mice lacking AT1 receptors did not develop age-related cerebral circulation damages.22
RAS inhibition in the liver of old rats enhanced gene levels of nuclear respiratory factor 1 and PPAR-gamma, which are involved in mitochondrial respiration and biogenesis, respectively. Therefore, RAS inhibition has maintained the integrity of the hepatocyte system and prevented liver fibrosis and the infiltration of inflammatory cells during aging.23
Sirtuins are NAD-dependent deacetylase proteins that are associated with longevity, mitochondrial and cell cycle regulation apoptosis, and DNA damage repair.7 In humans there are 7 different sirtuins (SIRT1-7), and 3 are located in the mitochondria (SIRT3-5). SIRT3 plays a very crucial role in extending life span. SIRT3 protects cardiomyocytes against Bax-mediated apoptosis by deacetylating Ku70. The deacetylated Ku70 binds to Bax and hence blocks Bax activation.24
SIRT3 exerts its actions only in the presence of the cosubstrate NAD+, and the concentration of NAD+ determines cell survival. Nicotinamide phosphoribosyltransferase (NAMPT) enhances mitochondrial NAD+ concentrations.
AT1 receptor-lacking mice showed increased levels of NAMPT and SIRT3 levels in their kidneys with respect to wild ones. Furthermore, candesartan, an AT1 receptor blocker, prevented Ang II-induced NAMPT and SIRT3 messenger RNA reduction in tubular epithelial cells, thereby enhancing cell survival.25 The longevity is the consequence of reduced mitochondrial damage due to attenuation of oxidative stress and upregulation of NAMPT and SIRT3 survival genes.
Resveratrol, a small molecule found in red wine, inhibits AT1 receptors, which contribute to resveratrol-induced longevity.26 Thus, the inhibition of AT1 receptors could represent a possible therapeutic strategy for diseases of aging and possibly even for extending life span.7
Functions of RAS and Alternate RAS In the Brain
Central Sympathetic System and Blood Pressure Control
The hypothalamic paraventricular nucleus (PVN) is the single most crucial center of the central autonomic network. The PVN also functions as the central neurocircuitry of the renal sympathetic nerve activity (RSNA) and cardiac sympathetic afferent (CSAR), controlling sympathetic activity through projections to the rostral ventrolateral medulla and the intermediolateral column of the spinal cord.
The main function of the PVN is to moderate tonic sympathetic activity, especially the CSAR and RSNA. Both endogenous Ang (1-7) and Ang II in the PVN enhance CSAR and RSNA. The Mas receptor is essential for Ang (1-7) stimulatory action in the PVN. Overexpression of ACE2 and consequently Ang (1-7) production in the PVN attenuated the Ang II-induced increase in blood pressure and production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, and IL-6, in the PVN. Therefore, the ACE2/Ang (1-7)/Mas axis results in anti-inflammatory and antihypertensive effects in the PVN.1,27 Ang (1-7) acts tonically in the nucleus tractus solitarius (NTS) to enhance the sensitivity of baroreflex-mediated changes in the heart rate of normotensive rats, and bradycardia in old rats was associated with the loss of an Ang (1-7) effect in NTS.28
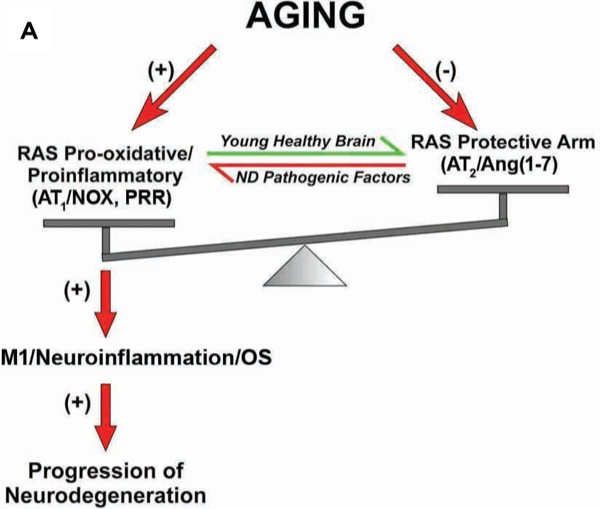
Antenatal steroid exposure is linked with hypertension during adolescence, possibly because tonic Ang II overcomes Ang (1-7) effects in NTS.2 Ang (1-7) restrains central sympathetic nerve activity via Mas receptor activation, which in turn enhances neuronal nitric oxide synthase (nNOS) and NO production. Increased NO production reduces the activity in catecholaminergic neurons. Collectively, there is an intricate equilibrium balancing Ang II and Ang (1-7) in the central nervous system (CNS) that normally maintains baroreceptor reflex and blood pressure. Aging is associated with predominance of Ang II effects, with the reduction of Ang (1-7) and ACE2 activities resulting in hypertension and other cardiovascular diseases.
Non-Cardiovascular Functions of ACE2/Ang (1-7)/Mas Axis in the Brain
Ang (1-7) via its action on Mas receptors enhances NO production through nNOS activation in the brain, which is a crucial factor for object recognition memory and long-term enhancement in the hippocampus and amygdala. Knockout Mas receptor rats had a deficit in object recognition memory, confirming the importance of the Ang (1-7)/Mas axis.29 Ang (1-7) may have an important neuroprotective role against cerebral ischemic stroke. Ang (1-7) acting via Mas receptors showed reduced cerebral infarct size and improved performance on neurologic examination in treated rats.30
Ang (1-7) enhances endothelial NOS (eNOS) activity and consequently cerebral blood flow to ischemic brain tissues. Ang (1-7) inhibits the inducible NOS (iNOS) activity. The increased production of NO by the enhanced activity of iNOS in the ischemic brain will enhance the concentration of peroxynitrite, a powerful oxidant, and may increase the tissue damage after cerebral ischemia. Ang (1-7) suppresses NF-kappa B via Mas receptors. Therefore, Ang (1-7) could be a useful neuroprotective factor via its anti-inflammatory effects after cerebral ischemia.31
The ACE2/Ang (1-7) axis has been detected in the glial cells of the human retina. Intravitreal injection with Ang (1-7) decreased intraocular pressure in rabbits. In addition, intraocular administration of ACE2 or Ang (1-7) genes in diabetic rats conferred protection against diabetic retinopathy.32
3-RAS and Microglia
During embryonic development, primitive yolk sac myeloid progenitors enter the brain and differentiate into microglial cells. It is usually estimated that around 10% of adult brain cells are microglia cells. Microglia can develop into pro-inflammatory/classically activated macrophages (M1) or anti-inflammatory activated macrophage (M2) phenotypes, depending on the signals present at different stages after brain lesions. M1/pro-inflammatory microglia produces pro-inflammatory mediators and ROS that exacerbate neuronal death. Alternatively, M2/immunoregulatory microglia induce brain repair and regeneration, and produce growth factors and anti-inflammatory cytokines to protect neurons and resolve inflammation.
Several subclasses of M2/immunoregulatory activation have been identified. The M2a activation state has a main function of suppression inflammation. A second state of alternative activation is classified as M2c, which has been suggested to restore the tissue after the inflammatory process has been attenuated. M2b has been involved in both pro- or anti-inflammatory responses and is related to memory immune responses. Collectively, M2 phenotype cells are involved in anti-inflammation, debris clearance, extracellular matrix deposition, and angiogenesis functions in the brain.
Progression from the pro-inflammatory/M1 to the immunoregulatory/M2 phenotype is necessary to efficiently counteract brain lesions. However, when this process is dysregulated, the persistent release of inflammatory cytokines and ROS induces neuron death and enhances brain damage. Persistently pro-inflammatory M1 microglia in the brain is the key factor for the development of neurodegenerative disorders including multiple sclerosis, Alzheimer’s disease, and parkinsonian syndromes. Of note, the FDA-approved drug glatiramer acetate, for multiple sclerosis treatment, works by inducing Th1 to Th2 shift, resulting in the production of anti-inflammatory cytokines such as IL-4 that polarize the microglia into the M2 anti-inflammatory phenotype.
Cytokines including TNF-alpha, IL-6, IL-1beta, and interferon-gamma as well as several chemokines, in addition to the level of microglia NADPH-oxidase activation, are essential to shift the microglia to pro-inflammatory type M1. Ang-II via its AT1 receptor is a major activator of the NADPH-oxidase complex, leading to pro-oxidative and pro-inflammatory effects resulting in the shift to the M1 type. However, anti-inflammatory cytokines such as IL-4, IL-10, and PPAR-gamma agonists polarize the microglia toward the anti-inflammatory M2 type.
The ARB group is heterogeneous, with some members—notably telmisartan and to a lesser extent candesartan—exhibiting a pleiotropic profile, not only blocking the AT1 receptor but also activating PPAR-gamma, an anti-inflammatory and pro-metabolic nuclear receptor, thereby helping to shift the microglia cells toward the anti-inflammatory M2 type.
Alzheimer’s disease is the most common form of dementia, characterized by the presence of neurofibrillary tangles of hyperphosphorylated tau and extracellular deposits of the peptide amyloid beta (Abeta), forming neuritic plaques. Another key feature of Alzheimer’s disease is the presence of prominent neuroinflammation. There are several ways for Abeta to be cleared from the brain. Abeta can be directly shuttled out of the brain via protein complexes, such as LRP1 and apolipoprotein E, which can bind extracellular Abeta and transport it to the blood–brain barrier, where it is then shuttled to the other side. Extracellular Abeta in central nervous system (CNS) interstitial fluid is moved into the cerebrospinal fluid via the newly discovered glymphatic pathway. Finally, Abeta can be cleared via phagocytosis and degradation by resident CNS immune cells, such as microglia, astrocytes, and possibly neurons. The M2 type is the key player in the process of phagocytosis and clearance of Abeta from the brain.33-36
The treatment with ARBs has the ability to shift the microglia toward the M2 type and thereby has improved cognition in many rodent models of Alzheimer’s disease, in doses that did not significantly lower blood pressure. Therefore, administration of ARBs to hypertensive patients reduced the risk not only for Alzheimer’s disease but also for vascular dementia.34 In controlled clinical trials, several ARBs not only limit stroke-induced damage, protecting executive function and cognition, but also reduce hypertension and diabetes, which are major risk factors for stroke.35-39
Parkinson’s disease is characterized by enhanced NADPH-oxidase activity, enhanced uncontrolled inflammatory processes, increased TNF-alpha production, regulation of alpha-synuclein, reduction of brain neurotrophic factors, and decreased activation of PPAR-gamma. The use of the most potent ARBs, candesartan and telmisartan, is considered to be among the new treatments for Parkinson’s disease.37
In vivo and in vitro studies revealed that the ARB olmesartan increased neurite outgrowth and acetyltransferase activity in primary cultures of the ventral spinal cord and enhanced survival of motor neurons after unilateral section of the sciatic nerve. Therefore, olmesartan is considered a possible therapeutic agent in disorders leading to degeneration of motor neurons, such as amyotrophic lateral sclerosis (Figure 2).36
Figure 2.
Ang IV and the AT4 Receptor
Ang IV effects occur via the AT4 receptor, which is widely distributed in the brain, including the neocortex, cerebellum, anterior pituitary, and many other brain areas. The AT4 receptor has been recognized as insulin-regulated aminopeptidase (IRAP). IRAP is a member of the M1 family of metallopeptidases that also contains aminopeptidases. IRAP splits the N-terminal amino acid from peptidases, such as vasopressin, oxytocin, somatostatin, eNOS, and many others. IRAP also inhibits intracellular insulin–regulated glucose transporter-4 in the pyramidal cells of the hippocampus and cerebral cortex. Ang IV is a natural inhibitor to IRAP. Therefore, Ang IV helps to improve glucose uptake and the availability of peptides essential to memory and cognition, such as oxytocin and vasopressin. In addition, it enhances the availability of eNOS, which is essential for cerebral blood flow.2,37-39 Of note, exenatide, the glucagon-like peptide-1 receptor (GLP-1R) agonist that increases glucose level–dependent insulin secretion, has been shown to improve motor and cognition functions in patients with Parkinson’s disease. Exenatide enhances insulin in the brain, and therefore it inhibits IRAP.38 In addition, the use of liraglutide, another GLP-1R agonist for nondiabetic patients with mood disorders, improves patients’ cognitive functions.39 A second subtype of the AT4 receptor has been reported to be the c-Met receptor for hepatocyte growth factor. Ang IV competes with hepatocyte growth factor for binding to c-Met, thereby inhibiting its actions.40,41
Cardiovascular Actions of the RAS
The harmful effects of the classic RAS on the cardiovascular system, resulting in hypertension and increased inflammation, are mediated by Ang II via the AT1 receptor, while the counter-regulatory axis of RAS, which acts mainly by the Ang (1-7)/AT2/Mas axis, produces vasodilation and anti-inflammatory, antithrombotic, and antiproliferative effects. Ang (1-7) enhances the release of NO by activating eNOS. Ang (1-7) modulates myocardium Ca++ handling by increasing NO and eNOS activity, leading to activation of the sarcoplasmic reticulum calcium ATPase 2a pump that transports calcium ions from the cytoplasm into the sarcoplasmic reticulum, thereby preventing the pathologic effects of Ca++ overload. In addition, it prevents left ventricular remodeling and interstitial fibrosis, and preserves cardiac functions.1
The mineralocorticoid receptor blocker eplerenone reduces oxidative stress by decreasing NADPH-oxidase and ACE activity, while it increases ACE2 activity, thereby increasing Ang (1-7) and decreasing the formation of Ang II. These results could explain the benefit of using mineralocorticoid receptor blockers in patients with congestive heart failure, which was demonstrated in studies called RALES (Randomized Aldactone Evaluation Study) and EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study).42-44Furthermore, use of the ARBs olmesartan and valsartan for 6 months was associated with reduced coronary plaque volume in patients with stable angina pectoris. The regression in coronary plaque volume is associated with reduced incidence of myocardial infraction and revascularization.43,44
RAS and the Kidney
ACE2 is considered the main source for Ang (1-7) in renal tissues. The alternate RAS play a very important role in protecting and preserving renal function. Ang (1-7) exerts both antidiuretic and diuretic effects in the proximal tubule, according to its concentration. Ang (1-7) at low concentrations (10–9 M) stimulates Na+-ATPase activity via AT1 receptors; however, at higher concentrations (10–6 M), it inhibits the Na-ATPase activity via AT2 receptors. Furthermore, at higher concentrations Ang (1-7) counteracts the stimulatory effect of Ang II on proximal tubule Na+-ATPase activity, an effect mediated by the Mas receptor. The Na+-ATPase enzyme is involved in fine-tuning and fast regulation of sodium reabsorption in the renal cortex.45-48
Excessive classic RAS activation results in high intrarenal Ang II levels and subsequent systemic and glomerular capillary hypertension, which can lead to endothelial injury and eventual kidney damage. Ang (1-7) vasodilates the preconstricted renal afferent arterioles and enhances renal blood flow. Ang (1-7) increases renal atrial natriuretic peptide production, which reduces oxidative stress, as well as inflammatory, fibrotic, and proliferative effects of Ang II in the kidney. Therefore, Ang (1-7) is considered to be an important physiologic regulator of intraglomerular pressure that opposes the harmful effects of excessive Ang II production. Ang (1-7) stimulates NO production via a prostaglandin-dependent vasodilatory pathway, which could be prevented by administration of indomethacin.46,47
Treatment with ACE inhibitors and ARBs is accompanied by elevated plasma levels of Ang (1-7), which may explain the mechanism of their therapeutic effects against the development of diabetic nephropathy and hypertension-induced kidney damage. Furthermore, blocking of AT1receptors on the juxtaglomerular cells of the kidney by ARBs will increase in renin release, with subsequent increases in the formation of Ang I and Ang II. The increased levels of circulating Ang II would then lead to a selective increase in AT2 receptor stimulation.48
The perioperative use of RAS antagonists may exert a protective effect against acute kidney injury. Intravenous enalapril at the induction of anesthesia in patients undergoing aortic surgery with infrarenal cross-clamping was associated with enhanced systemic oxygen delivery, improved splanchnic circulation, and an improved glomerular filtration rate 24 hours after surgery.49
Diabetic Nephropathy
High glucose concentration associated with diabetes along with increased intracellular ROS induces Ang II formation in podocytes. Ang II acts on AT1 receptors to further enhance ROS generation and transforming growth factor (TGF)-beta1 generation. Through these mechanisms, it enhances podocyte loss, glomerulosclerosis, and interstitial fibrosis.50 Ang (1-7) reduced proteinuria and vascular activity in isolated renal artery segments in diabetic rats. ACE2 overexpression in podocytes could delay and ameliorate the development of glomerulopathy in type 1 diabetes, indicating that ACE2 may slow the development of diabetic nephropathy via glomerular direct effects.51
Role of RAS in Liver Fibrosis
Liver fibrosis results from a complex pathologic interaction between hepatic stellate cells, Kupffer cells, cytokines, chemokines, and growth factors. Stellate cells are also called lipid storage cells, lipocytes, or Ito cells, which lie in the space of Disse, the subendothelial space between hepatocytes and sinusoidal endothelial cells. The main function of hepatic stellate cells is to metabolize vitamin A and produce cytokines, growth factors, and inflammatory mediators. In addition, hepatic stellate cells play a vital role in the regulation of portal pressure. Hepatic stellate cells can transform into myofibroblasts in response to chronic liver injury and have the ability to contract scar tissue and fibrous septa.52 After hepatic injury, TGF-beta, cytokines, and ROS released from hepatocytes, Kupffer cells, and inflammatory cells induce the change of hepatic stellate cells into myofibroblasts. Activated hepatic stellate cells produce extracellular matrix proteins (collagen I and III), matrix metalloproteinases (MMPs), and their respective tissue inhibitors. The secretion of MMPs and their inhibitors indicates that fibrosis may be reversible conditionally.
After liver injury, Ang II via AT1 receptors induces contraction and proliferation of hepatic stellate cells, increasing hepatic acinar fibrosis. In addition, the Ang II/AT1 receptor axis enhances inflammation and ROS production in the injured liver. The Ang (1-7)/Mas axis has a protective effect against liver fibrosis. The use of ACE inhibitors and ARBs was successful in preventing fatty liver disease and improved fibrosis in obese Zucker rats, with a reduction in the hepatic expression of the profibrotic TNF-alpha and TGF-beta1. Despite the beneficial effects of ACE inhibitors and ARBs, they failed to reduce hepatic fibrosis in patients with chronic hepatitis C.53On the other hand, RAS blockade was associated with reduction of hepatic inflammation and fibrosis due to hepatitis C in post–liver transplant patients.54,55
RAS and Alternate RAS in Lung Pathophysiology
The lung is one of the major sources for systemic Ang II. Because the ACE D/D genotype is associated with high levels of Ang II, increased ROS, inflammation, and fibrosis, it is not surprising that in patients with chronic obstructive pulmonary disease, the ACE D/D genotype was associated with a higher incidence of impaired peripheral tissue oxygenation and unfavorable pulmonary complications compared with ACE D/I or ACE I/I.56
Ang II is a bronchoconstrictor implicated in the development of asthma, an effect that can be reversed with Ang (1-7). ACE2/Ang (1-7) has protective effects on pulmonary functions, as it exerts vasodilator, antifibrotic, antiproliferative, and anti-inflammatory effects in pulmonary circulation.57 Furthermore, ACE2 has been identified as a receptor for severe acute respiratory syndrome in in vitro cell lines. ACE2 binds to coronal virus spike proteins, the etiologic agent of this syndrome, resulting in the downregulation of ACE2 expression, possibly explaining the progression of the disease to acute respiratory distress syndrome.58 Recently, an antiparasitic agent, diminazene, was also found to enhance ACE2 activity and attenuate experimental pulmonary hypertension in rats.59
Preeclampsia and RAS
Preeclampsia is the second-leading cause of maternal mortality in the United States, affecting 7% to 10% of all pregnancies, resulting in stillbirth and neonatal morbidity and mortality.60 In preeclampsia autoantibodies are found, and they function as agonists of the AT1 receptors in the placenta.61 The chorionic villi of preeclamptic women was shown to have higher than normal Ang (1-7) levels when compared with those from women without preeclampsia. Therefore, the increased Ang II levels in preeclamptic chorionic villi may contribute to the pathophysiology of preeclampsia by decreasing the maternal–fetal exchange of vital oxygen and nutrients.62
Antiangiogenesis Effects of Ang (1-7)
Ang (1-7) inhibits vascular endothelial growth factor, thereby reducing blood vessel density and tumor cell proliferation. Moreover, Ang (1-7) inhibits cyclooxygenase-2 enzyme activity, which plays an important role in tumor growth and metastasis. In a retrospective cohort study of over 5,000 Scottish individuals, the use of ACE inhibitors was associated with significantly greater cancer-free survival and survival without fatal cancer than other antihypertensive drugs, especially with sex-specific cancers in women and smoking-related cancers.63
Preoperative Administration of RAS Antagonists
As of 2012, RAS antagonists—either ACE inhibitors or ARBs—were used by approximately 18% of adults in the United States and by 43% of veterans for major surgery.64 RAS antagonists are considered the mainstay treatment in patients with cardiovascular disease and those with diabetes. Therefore, the current guidelines from the American College of Cardiology and American Heart Association recommend continuing ACE inhibitors/ARBs in the setting of noncardiac surgery.65
Recently, Roshanov et al used the large, multinational, prospective VISION data to examine risk-adjusted associations of ACE inhibitor/ARB administration.66 The primary outcome of the VISION study was to evaluate associations of early postoperative troponin T release with 30-day mortality in patients over 45 years of age requiring at least an overnight stay in the hospital. The subgroup analysis focused only on 33% of VISION patients who have been treated with RAS antagonists preoperatively (n=4,802), of whom 26% did not receive a dose within 24 hours of surgery (n=1,245). Findings of the study suggest that withholding RAS antagonists before major noncardiac surgery was associated with less risk for complications and death caused by hypotension developing during or shortly after induction of anesthesia; however, the incidence of perioperative myocardial infarction did not differ significantly between the 2 groups.
The editorial by London accompanying the study stated that although ACE inhibitor/ARB use was associated with intraoperative hypotension and progressively longer duration, it was not associated with the outcome. Postoperative hypotension was associated with the primary outcome but not with ACE inhibitor/ARB use.64 The study has limitations, such as the lack of randomization, varying types of RAS antagonists, varying dosages given, and no controls for age and comorbidities.67 Moreover, Lee et al showed that failure to resume ARBs within 2 days in the postoperative period was associated with a 30-day mortality rate of 3.2%, versus 1.3% for those who resumed ARB medications.68
According to a recent meta-analysis, patients who received RAS antagonists on the day of surgery did not have an increased incidence of postoperative complications such as myocardial infarction, stroke, acute kidney injury, and death. Therefore, there was insufficient evidence to support withholding RAS antagonists on the day of surgery. However, the authors cautioned about the risk for intraoperative hypotension in patients receiving RAS antagonists, which should be treated effectively.69 Furthermore, the continuation of RAS antagonist use in cardiac surgery patients might provide benefits for patients with diabetes.70
The new discoveries of ARBs as neuroprotective agents have made these agents helpful tools in perioperative medicine, especially in elderly patients. Therefore, the decision to continue or discontinue RAS antagonists, especially ARBs, should be individualized according to patient comorbidities, and hypotension should be properly managed to ensure appropriate tissue perfusion.
Conclusion
New discoveries involving the classic and alternate RAS pathways, as well as insights concerning the use of ARBs, open new opportunities for treating a variety of conditions. These developments have the potential to improve the perioperative care and outcomes of critically ill patients.
References
- Farag E, Maheshwari, K, Morgan J, et al. An update of the role of renin angiotensin in cardiovascular homeostasis. Anesth Analg. 2015;120(2):275-292.
- Farag E, Sessler D, Ebrahim Z, et al. The renin angiotensin system and the brain: new developments. J Clin Neurosci. 2017;46:1-8.
- Dell’Italia L. Translational success stories: angiotensin receptor 1 antagonists in heart failure. Circ Res. 2011;109(4):437-452.
- Lorenz JN. Chymase: the other ACE? Am J Physiol Renal Physiol. 2010;298(1):F35-F36.
- Park S, Bivona BJ, Kobori H, et al. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298(1):F37-F48.
- Rush JW, Aultman CD. Vascular biology of angiotensin and the impact of physical activity. Appl Physiol Nutr Metab. 2008;33(1):162-172.
- Cassis P, Conti S, Remuzzi G, et al. Angiotensin receptors as determinants of life span. Pflugers Arch. 2010;459(2):325-332.
- Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor appaB. Free Radic Biol Med. 2000;28(9):1317-1327.
- Kaparianos A, Argyropoulou E. Local renin-angiotensin II systems, angiotensin-converting enzyme and its homologue ACE2: their potential role in the pathogenesis of chronic obstructive pulmonary diseases, pulmonary hypertension and acute respiratory distress syndrome. Curr Med Chem. 2011;18(23):3506-3515.
- Hildeman DA, Mitchell T, Aronow B, et al. Control of Bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci USA. 2003;100(25):15035-15040.
- Horiuchi M, Iwanami J, Mogi M. Regulation of angiotensin II receptors beyond the classical pathway. Clin Sci. 2012;123(4):193-203.
- Iwanami J, Mogi M, Tsukuda K, et al. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-activation in diabetic mice. J Hypertens. 2010;28(8):1730-1737.
- Tsukuda K, Mogi M, Iwanami J, et al. Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009;54(4):782-787.
- Lindholm LH, Ibsen H, Dahlöf B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):1004-1010.
- Fujita T, Mogi M, Min LJ, et al. Attenuation of cuff-induced neointimal formation by overexpression of angiotensin II type 2 receptor-interacting protein 1. Hypertension. 2009;53(4):688-693.
- Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100(14):8258-8263.
- Jankowski V, Tölle M, Santos RA, et al. Angioprotectin: an angiotensin II-like peptide causing vasodilatory effects. FASEB J. 2011;25(9):2987-2995.
- Wilkinson-Berka J. Prorenin and the (pro)renin receptor in ocular pathology. Am J Pathol. 2008;173(6):1591-1594.
- Nguyen G, Delarue F, Burcklé C, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417-1427.
- Reudelhuber TL. Prorenin, renin, and their receptor: moving targets. Hypertension. 2010;55(5):1071-1074.
- Herbert KE, Mistry Y, Hastings R, et al. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008;102(2):201-208.
- Modrick ML, Didion SP, Sigmund CD, et al. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2009;296(6):H1914-H1919.
- de Cavanagh EM, Flores I, Ferder M, et al. Renin–angiotensin system inhibitors protect against age-related changes in rat liver mitochondrial DNA content and gene expression. Exp Gerontol. 2008;43(10):919-928.
- Sundaresan NR, Samant SA, Pillai VB, et al. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28(20):6384-6401.
- Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type receptor promotes longevity in mice. J Clin Invest. 2009;119(3):524-530.
- Miyazaki R, Ichiki T, Hashimoto T, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28(7):1263-1269.
- Sriramula S, Cardinale JP, Lazartigues E, et al. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92(3):401-408.
- Sakima A, Averill DB, Gallagher PE, et al. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1-7) at the nucleus tractus solitarii. Hypertension. 2005;46(2):333-340.
- Lazaroni TL, Raslan AC, Fontes WR, et al. Angiotensin-(1–7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol Learn Mem. 2012;97(1):113-123.
- Mecca AP, Regenhardt RW, O’Connor TE, et al. Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke. Exp Physiol. 2011;96(10):1084-1096.
- Jiang T, Gao L, Guo J, et al. Suppressing inflammation by inhibiting the NF-b pathway contributes to the neuroprotective effect of angiotensin-(1-7) in rats with permanent cerebral ischaemia. Br J Pharmacol. 2012;167(7):1520-1532.
- Verma A, Shan Z, Lei B, et al. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol Ther.2012;20(1):28-36.
- Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98.
- Saavedra JM. Beneficial effects of angiotensin II receptor blockers in brain disorders. Pharmacol Res. 2017 Nov;125:91-103.
- Davies NM, Kehoe PG, Ben-Shlomo Y, et al. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011;26(4):699-708.
- Iwasaki Y, Ichikawa Y, Igarashi O, et al. Trophic effect of Olmesartan, a novel AT1R antagonist, on spinal motor neuronsin vitroandin vivo. Neurol Res. 2002;24(5):468-472.
- Wright JW, Harding JW. Brain renin-angiotensin—a new look at an old system. Prog Neurobiol. 2011;95(1):49-67.
- Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664-1675.
- Mansur RB, Ahmed J, Cha DS, et al. Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: a pilot, open-label study. J Affect Disord. 2017;207:114-120.
- Yamamoto BJ, Elias PD, Masino JA, et al. The angiotensin IV analog Nle-Tyr-Leu-psi-(CH2-NH2)3-4-His-Pro-Phe (norleual) can act as a hepatocyte growth factor/c-Met inhibitor. J Pharmacol Exp Ther. 2010;333(1):161-173.
- Kawas, LH, Yamamoto BJ, Wright JW, et al. Mimics of the dimerization domain of hepatocyte growth factor exhibit anti-Met and anticancer activity. J Pharmacol Exp Ther. 2011;339(2):509-518.
- Keidar S, Gamliel-Lazarovich A, Kaplan M, et al. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97(9):946-953.
- D’Ascenzo F, Agostoni P, Abbate A, et al. Atherosclerotic coronary plaque regression and the risk of adverse cardiovascular events: a meta-regression of randomized clinical trials. Atherosclerosis. 2013;226(1):178-185.
- Ishii H, Kobayashi M, Kurebayashi N, et al. Impact of angiotensin II receptor blocker therapy (olmesartan or valsartan) on coronary atherosclerotic plaque volume measured by intravascular ultrasound in patients with stable angina pectoris. Am J Cardiol. 2013;112(3):363-368.
- Caruso-Neves C, Lara LS, Rangel LB, et al. Angiotensin-(1–7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta. 2000;1467(1):189-197.
- Bernardi S, Burns WC, Toffoli B, et al. Angiotensin-converting enzyme 2 regulates renal atrial natriuretic peptide through angiotensin-(1–7). Clin Sci. 2012;123(1):29-37.
- Pinheiro SVB, Sim’es e Silva AC. Angiotensin converting enzyme 2, angiotensin-(1–7), and receptor mas axis in the kidney. Int J Hypertens. 2012;2012:1-8.
- Speth RC, Giese MJ. Update on the renin-angiotensin system. J Pharmacol Clin Toxicol. 2013;1:1004.
- Licker M, Bednarkiewicz M, Neidhart P, et al. Preoperative inhibition of angiotensin-converting enzyme improves systemic and renal haemodynamic changes during aortic abdominal surgery. Br J Anaesth. 1996;76(5):632-639.
- Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106(2):26-31.
- Nadarajah R, Milagres R, Dilauro, M, et al. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int. 2012;82(3):292-303.
- Pereira RM, dos Santos RA, da Costa Dias FL, et al. Renin-angiotensin system in the pathogenesis of liver fibrosis. World J Gastroenterol. 2009;15(21):2579–2586.
- Abu Dayyeh BK, Yang M, Dienstag JL, et al. The effects of angiotensin blocking agents on the progression of liver fibrosis in the HALT-C Trial cohort. Dig Dis Sci. 2011;56(2):564-568.
- Cholongitas E, Vibhakorn S, Lodato F, et al. Angiotensin II antagonists in patients with recurrent hepatitis C virus infection after liver transplantation. Liver Int. 2010;30(2):334-335.
- Rimola A, Londoño MC, Guevara G, et al. Beneficial effect of angiotensin-blocking agents on graft fibrosis in hepatitis C recurrence after liver transplantation. Transplantation. 2004;78(5):686-691.
- Kaparianos A, Argyropoulou E. Local renin-angiotensin II systems, angiotensin-converting enzyme and its homologue ACE2: their potential role in the pathogenesis of chronic obstructive pulmonary diseases, pulmonary hypertension and acute respiratory distress syndrome. Curr Med Chem. 2011;18(23):3506-3515.
- Li N, Cai R, Niu, Y, et al. Inhibition of angiotensin II-induced contraction of human airway smooth muscle cells by angiotensin-(1-7) via downregulation of the Rhoa/ROCK2 signaling pathway. Int J Mol Med. 2012;30(4):811-818.
- Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875-879.
- Shenoy V, Gjymishka A, Jarajapu YP, et al. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013;187(6):648-657.
- Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–952.
- Yia Y, Wne H, Bobst S, et al. Maternal autoantibodies from preeclamptic patients active angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 2003;10(2):82-93.
- Anton L, Merrill DC, Neves LA, et al. Activation of local chorionic villi angiotensin II levels but not angiotensin (1-7) in preeclampsia. Hypertension. 2008;51(4):1066-1072.
- Lever AF, Hole DJ, Gillis CR, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352(9123):179-184.
- London MJ. Preoperative administration of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers: do we have enough “VISION” to stop it? Anesthesiology. 2017;126(1):1-3.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College Of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):2215-2245.
- Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery. An analysis of the vascular events in noncardiac surgery patIents cOhort evaluatioN Prospective Cohort. Anesthesiology. 2017;126(1):16–27.
- Bradic N, Povsic-Cevra Z. Surgery and discontinuation of angiotensin converting enzyme inhibitors: current perspectives. Curr Opin Anaesthesiol. 2018;31(1):50-54.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology. 2015;123(2):288-306.
- Ling Q, Gu Y, Chen J, et al. Consequences of continuing renin angiotensin aldosterone system antagonists in the preoperative period: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18(1):26.
- Cheng X, Tong J, Hu Q, et al. Meta-analysis of the effects of preoperative renin-angiotensin system inhibitor therapy on major adverse cardiac events in patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2015;47(6):958-966.