INTRODUCTION
Remimazolam besylate (ByFavo™ in the USA and in South Korea, Anerem® in Japan, Aptimyda™ in EU, and Ruima® in China) is an intravenous, short-acting, and ultrafast onset benzodiazepine (nonanalgesic) with potent sedative-hypnotic, anxiolytic, anticonvulsant, and muscle relaxant properties. As its name suggests, the makers of the drug have attempted to combine the familiarity and therapeutic effects of midazolam with the unique metabolism of remifentanil.
So far, remimazolam has found a clinically impactful role in procedural sedation in Asia and Europe since its release in China in 2019 for use in gastrointestinal endoscopy. In Japan and Korea, its use has now been approved for general anesthesia, and, in Belgium, remimazolam was used for ICU sedation.1,2 In the United States, the FDA approved remimazolam for induction and maintenance of sedation in adults undergoing procedures lasting 30 minutes or less in July 20203 with non-FDA-approved uses being reported extensively in the literature. Despite this, few centers have acquired the drug, formulated internal guidelines for its use, and applied it to a large clinical practice.
As of publication of this article, our institution, the Mayo Clinic, is one of the first major academic centers in the United States to widely adopt remimazolam into the perioperative and periprocedural practice. We have used it in over 5,000 patients with over 20,000 doses administered. We are in the process of investigating definite clinical areas where remimazolam has an important practice-changing role including possibilities for safe clinical expansion.
In this review, we combine the available literature with our institutional experience on remimazolam’s patient safety profile amongst various clinical practices. We specifically discuss the unique pharmacokinetics and pharmacodynamics of remimazolam and outline some important nuances about its known limitations, adverse advents, and contraindication for use. We summarize key clinical practice implications and elucidate important knowledge gaps for its safe, widespread adoption including its role in anesthesia as well as nurse-driven sedation. We conclude with lessons learned regarding what is known and unknown about remimazolam’ s meaningful clinical outcomes, effects on practice efficiency, and patient safety profile.
PHARMACOLOGY: A BRIEF REVIEW
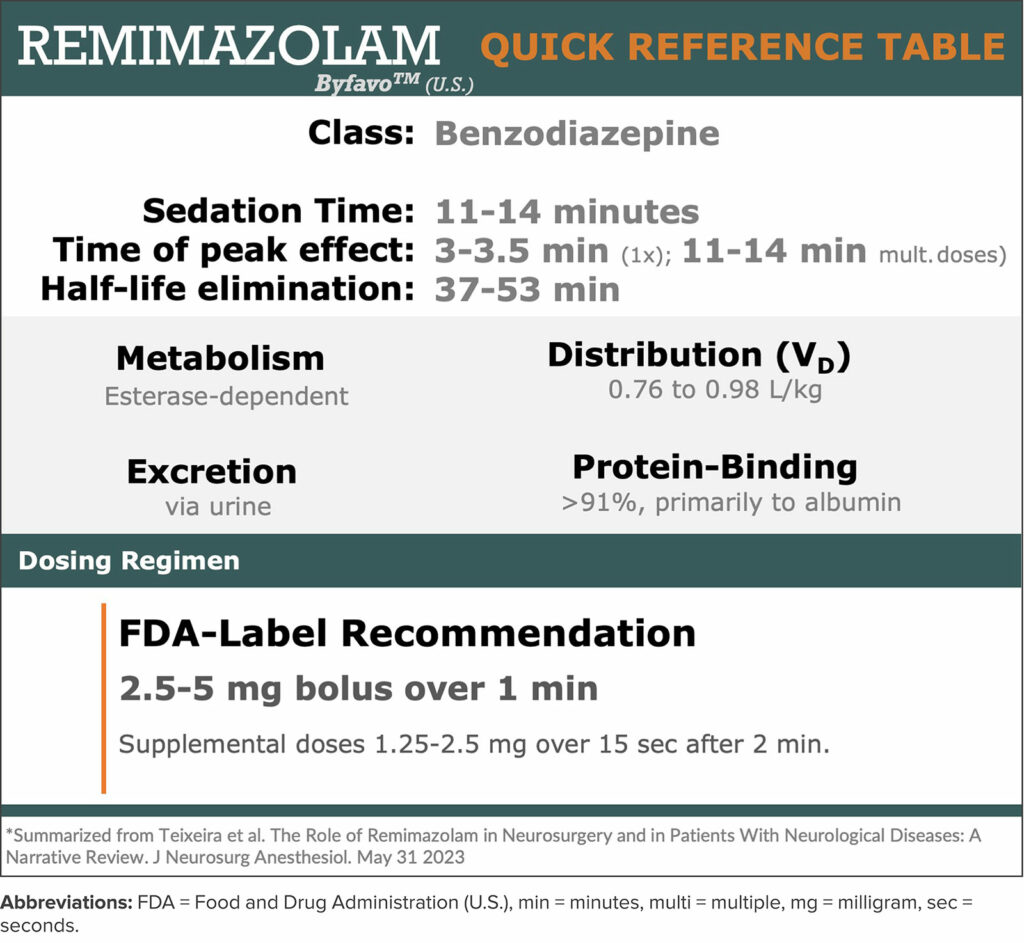
Remimazolam’ s mechanism of action is comparable to other benzodiazepines in that it enhances the gamma-aminobutyric acid type A (GABAA) inhibitory receptor leading to increased frequency of opening of ligand-gated chloride ion channels. It has desirable pharmacodynamics and exhibits minimal cardiac or respiratory depression. It has faster onset and dose dependent sedation than midazolam4 and is approximately half as potent5 for procedural sedation (Table 1). Like other benzodiazepines, its sedative effects can be reversed using flumazenil which have comparable active durations of effect.
Table 1: Quick Reference Guide for pharmacology and dosing for remimazolam.
From a pharmacokinetic standpoint, remimazolam has relatively high clearance, a small steady-state volume of distribution, shorter elimination half-life, and a short context sensitive half time compared to other benzodiazepines or propofol.6 Remimazolam is highly bound to protein and extensively metabolized primarily by liver carboxylesterase being excreted primarily in the urine.7,8 As such its structural modifications are similar to remifentanil in that it is a faster onset, titratable shorter-acting benzodiazepine.7
Remimazolam is water-soluble and when diluted into a solution becomes a painless injectate. It is most soluble in slightly acidic environments and can precipitate in lactated or acetated Ringers solution (Figure 1).9,10 It is compatible with y-site co-administration of common anesthetic drugs including remifentanil, fentanyl, dexmedetomidine, midazolam, and common neuromuscular blockers such as rocuronium and vecuronium.11 Currently, ByFavo® remimazolam is prepared in a 20 mg powder vial which is meant to be drawn up into 8.2 mL sterile 0.9% sodium chloride, making it 2.5 mg/ml after being reconstituted. The FDA labeling recommends 2.5–5 mg push injection over a one-minute time period followed by supplemental doses of two 1.25–2.5 mg aliquots intravenously over a 15-second time period after at least two minutes.3 In our experience, for procedural sedation, we typically dose 2 mg IV every 15 seconds as needed with or without analgesic adjuncts including ketamine or opiates (Figure 2). For induction of general anesthesia, we have employed a 0.2–0.4 mg/kg induction dose followed by 1–2 mg/kg/hr (Figure 2).12 Remimazolam has very low bioavailability (<2%).8
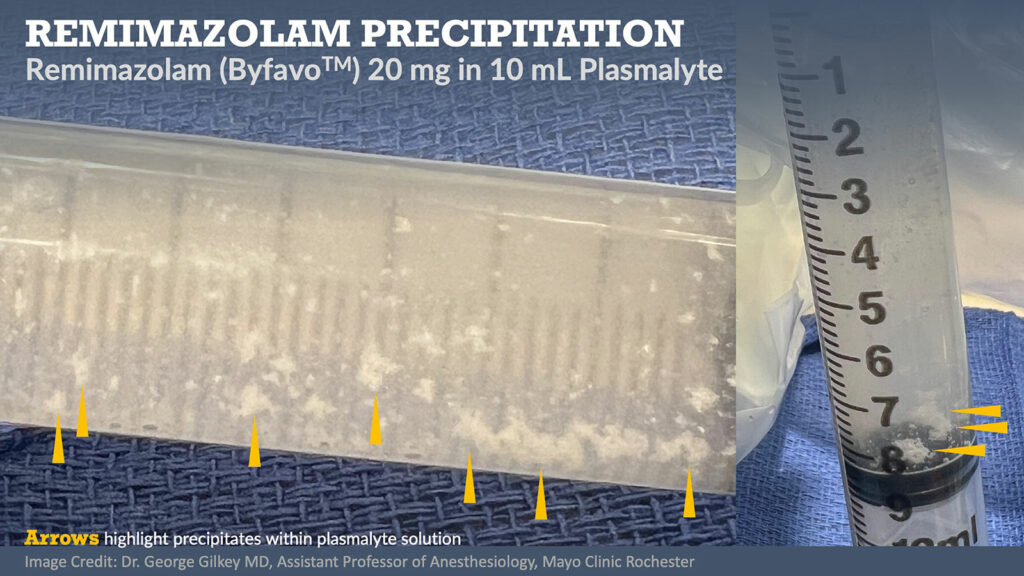
Figure 1: Photograph of 20 mg remimazolam (ByFavo™) drawn into 12 mL syringe of 10 mL plasmalyte. Yellow arrows highlight precipitate formation.
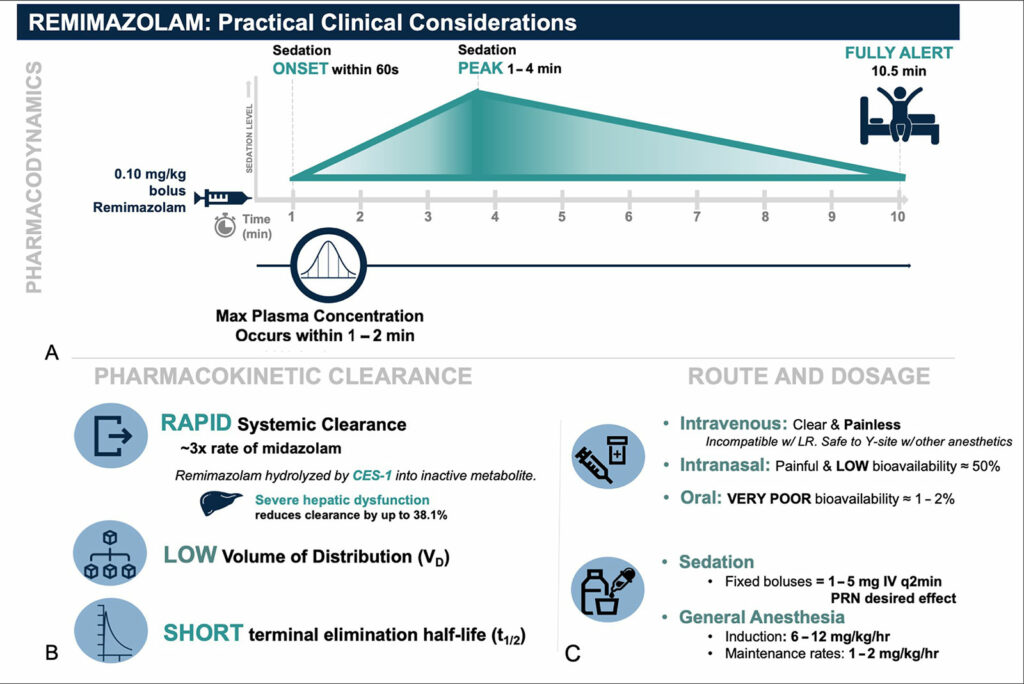
Figure 2: Remimazolam pharmacokinetic and pharmacokinetic profile adapted with permission from Figure 2 from Teixeira et al. “The role of remimazolam in neurosurgery and in patients with neurological diseases: a narrative review.” J Neurosurg Anesthesiol, May 31, 2023.
Abbreviations: mg = milligram, kg = kilogram, hr = hour.
UNKNOWN PATIENT SAFETY CONSIDERATIONS FOR REMIMAZOLAM
Remimazolam appears to be a relatively safe medication. However, we likely do not fully understand the impact of remimazolam on clinical outcomes after specific surgeries or procedures or within specific patient populations. Given its relative novelty and limited clinical use thus far, we advise continued caution recognizing that much remains unknown. Reporting unexpected serious adverse events is encouraged. Some patient safety considerations or questions that should be elucidated are as follows:
- Recovery in neurologically vulnerable patients: Most common benzodiazepines are considered to promote the development of delirium. Therefore, they should be administered with caution in neurologically vulnerable patients, particularly in the elderly. Current studies describing postoperative delirium after remimazolam are limited and likely not generalizable to larger populations or procedure types. Furthermore, the relationship between remimazolam administration and long-term postoperative neurocognitive disorder has not been established. We have described the most recent literature on remimazolam in a recent JNA 2023 review article (Figure 2).13,14
- Adverse reactions in specific patient populations and surgical subtypes: The pharmacokinetic properties of remimazolam appear to not be significantly altered in elderly or those patients with higher ASA scores.15 We follow the FDA recommendations for slight dose reduction and will also reduce the dose in those with severe hepatic impairment (Child-Pugh score ≥10) as they appear to have reduced drug clearance.16 No dose adjustments are needed for those with renal disease.16 Currently there is no pediatric labeling for general anesthesia or sedation, but off-label case reports of its use, predominately as an adjunct to general anesthesia, have been summarized in the literature.17 We have not found any reported cases of its use in pregnant patients.
- Administration and practice guided by nonanesthesia professionals: Midazolam is a commonly administered drug by periprocedural nursing staff. While gastrointestinal endoscopic studies have described safe use of remimazolam by nonanesthesia professionals, we have found adapting to primary-remimazolam from a primary-midazolam sedative nursing practice can take significant time, training, and cultural shifts.
- Cost and Access: Currently, remimazolam is invariably more expensive than more common sedative medications like midazolam and propofol. However, faster recovery times may facilitate increased procedural throughput and counter increased costs.5,18
ADVERSE REACTIONS AND CONTRAINDICATIONS
Overall, remimazolam appears to be a safe anesthetic drug as its adverse reactions tend to be mild, short-lived, and reversible by a single dose of flumazenil. Despite its relatively short context-sensitive half-life, care must be taken to ensure adequate reversal in those with prolonged infusions, patients with significant liver disease, and congruent opioid administration. Resedation from remimazolam after reversal with flumazenil is unlikely but has been reported.19
Common adverse reactions include blood pressure and heart rate variations, body movement, nausea, dizziness, and headaches.2,3 To put these into context, when compared to propofol, these risks have been reported as less likely, but similar to midazolam use.20,21 Importantly, remimazolam when co-administered with other central nervous system depressants including opioids, may lead to synergistic effects and may lead to significant respiratory depression. Also, anaphylaxis is possible and has been reported.22 The use of remimazolam is specifically contraindicated in patients with known severe hypersensitivity reactions to Dextran 40.3
There are early conflicting and limited data regarding remimazolam and its potential link to postoperative nausea and vomiting. It likely leads to a reduction in the incidence of postoperative nausea and vomiting when compared to volatile anesthesia alone,23 but not when compared to propofol.20
Complex Cardiovascular or Hemodynamically Unstable Patients:
Anecdotally, at our institution, remimazolam has quickly found a significant role in almost every area of practice—particularly in clinical areas with more clinically complex patients and procedures. Here are specific clinical areas where remimazolam has found a significant role in our practice and within the medical literature:
Remimazolam has limited impact on respiratory depression, systemic vascular tone, and inotropic, dromotropic, or chronotropic function. Therefore, many anesthesia professionals in our practice use it in the cardiac catheterization lab (routinely for cardioversions) and during cardiac surgery and trauma cases in patients with limited cardiopulmonary reserve.24
- Non-Operating Room Anesthesia (NORA)
- GI and Pulmonary Endoscopic Procedures: Some of the largest body of literature for remimazolam exists in endoscopy at this time. These trials have shown comparable efficacy for procedural sedation with a safety profile notable for fewer effects on hemodynamic function, a lack of pain with intravenous administration, reduction of postprocedure nausea and vomiting, and a rapid return to baseline neurologic function. 22,25,26
- Interventional Radiology: Patients requiring sedation under anesthesia care for interventional radiology often carry complex comorbidities, require deeper levels of sedation, or are too unstable for open surgical management. Moreover, these procedures often have limited, intermittent periods of discomfort. Remimazolam may have a significant role in providing sedation, amnesia, and anxiolysis during these procedures.
- Magnetic Resonance Imaging (MRI): Some patients require anesthesia support for MRI due to a variety of reasons, (e.g., claustrophobia, musculoskeletal discomfort, tremors, etc.) Remimazolam, in some patients, has been a uniquely valuable tool for MRI sedation. Anesthesia professionals have also used dexmedetomidine in conjunction with remimazolam for monitored anesthesia care in the MRI environment.27 In some patients with back pain, specifically central spinal cord stenosis, we are concerned that supine positioning under anesthesia could lead to prolonged or permanent spinal cord ischemia. Intermittent remimazolam boluses for sedation have allowed us to achieve a level of sedation adequate for accurate scans while at the same time, permitting intermittent neurologic exams. Small doses can provide enough patient anxiolysis while maintaining a patent airway to complete a brain MRI. At the time this article was written, we do not formally have nurses performing sedation with remimazolam.
- Neurosurgical Procedures: We recently reviewed our institution’s use of remimazolam in neurosurgery14 including its known effects on neuromonitoring and processed EEG. Particularly advantageous in neurosurgery, the pharmacokinetics and pharmacodynamics of remimazolam allow for rapid amnestic sedation and anxiolysis followed closely by a rapid meaningful neurologic exam. As such, we have used remimazolam for the following procedures: awake craniotomies for periods of discomfort during pin placement, local anesthetic administration, urethral catheter placement, and surgical incision.
LOOKING AHEAD: REMIMAZOLAM’ S IMPACT ON PERIOPERATIVE PATIENT SAFETY
In the two years of clinical experience with remimazolam, we have seen rapid expansion of its clinical use. This is likely attributed to its attractive pharmacokinetics, relative respiratory and hemodynamic safety profile, and its ability to be rapidly reversed. We predict this trend will continue as we expand its use to nurse sedation practice, especially within outpatient and ambulatory settings. Anesthesia professionals have a unique opportunity to identify patient safety practice guidelines, clinical guardrails, and safety algorithms for remimazolam. More large patient cohort safety data are forthcoming to truly delineate its safety profile compared to the other commonly used sedatives in the anesthesia professionals’ arsenal.
REFERENCES
- Keam SJ. Remimazolam: first approval. Drugs. 2020;80:625–633. PMID: 32274703
- Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. PMID: 34354587
- FDA. BYFAVO™ (remimazolam) FDA packet insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212295s000lbl.pdf Accessed August 8, 2023
- Rogers WK, McDowell TS. Remimazolam, a short-acting GABA(A) receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures. IDrugs. 2010;13:929–937. PMID: ~:text=Remimazolam%20(CNS%2D7056)%20is,induction%20and%20maintenance%20of%20anesthesia.”>21154153
- Dao VA, Schippers F, Stöhr T. Efficacy of remimazolam versus midazolam for procedural sedation: post hoc integrated analyses of three phase 3 clinical trials. Endosc Int Open. 2022;10:E378–e385. PMID: 35433203
- Kim KM. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med (Seoul). 2022;17:1–11. PMID: 35139608
- Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–66. PMID: 17585216
- Pesic M, Stöhr T, Ossig J, et al. Remimazolam has low oral bioavailability and no potential for misuse in drug-facilitated sexual assaults, with or without alcohol: results from two randomised clinical trials. Drugs R D. 2020;20:267–277. PMID: 32757149
- Yoshida K, Tanaka S, Watanabe K. A case of intravenous line occlusion when using Acetated Ringer’s solution and remimazolam. J Clin Anesth. 2021;70:110190. PMID: 33571823
- Sasaki H, Hoshijima H, Mizuta K. Ringer’s acetate solution-induced precipitation of remimazolam. Br J Anaesth. 2021;126:e87–e89. PMID: 33358048
- Kondo M, Yoshida N, Yoshida M, et al. Physical compatibility of remimazolam with opioid analgesics, sedatives, and muscle relaxants during simulated Y-site administration. Am J Health Syst Pharm. 2023;80:e53–e58. PMID: 36094564
- Lee HC. Remimazolam: another option for induction of general anesthesia? Korean J Anesthesiol. 2022;75:457–459. PMID: 36464845
- Schüttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and Pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132:636–651. PMID: 31972655
- Teixeira MT, Brinkman NJ, Pasternak JJ, Abcejo AS. The role of remimazolam in neurosurgery and in patients with neurological diseases: a narrative review. J Neurosurg Anesthesiol. 2023;doi:10.1097/ana.0000000000000917 PMID: 37264540 Online ahead of print.
- Doi M, Hirata N, Suzuki T, et al. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34:491–501. PMID: 32303884
- Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127:415–423. PMID: 34246461
- Kimoto Y, Hirano T, Kuratani N, et al. Remimazolam as an adjunct to general anesthesia in children: adverse events and outcomes in a large cohort of 418 cases. J Clin Med. 2023;12 PMID: 37373624
- Pedersen MH, Danø A, Englev E, et al. Economic benefits of remimazolam compared to midazolam and propofol for procedural sedation in colonoscopies and bronchoscopies. Curr Med Res Opin. 2023;39:691–699. PMID: 36999319
- Yamamoto T, Kurabe M, Kamiya Y. Re-sleeping after reversal of remimazolam by flumazenil. J Anesth. 2021;35:322. PMID: 33687549
- Doi M, Morita K, Takeda J, er al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth.2020;34:543–553. PMID: 32417976
- Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155:137–146. PMID: 30292760
- Tsurumi K, Takahashi S, Hiramoto Y, et al. Remimazolam anaphylaxis during anesthesia induction. J Anesth. 2021;35:571–575. PMID: 34050439
- Hari Y, Satomi S, Murakami C, et al. Remimazolam decreased the incidence of early postoperative nausea and vomiting compared to desflurane after laparoscopic gynecological surgery. J Anesth. 2022;36:265–269.PMID: 35142931
- Nakanishi T, Sento Y, Kamimura Y, et al. Remimazolam for induction of anesthesia in elderly patients with severe aortic stenosis: a prospective, observational pilot study. BMC Anesthesiol. 2021;21:306. PMID: 34872518
- Chen S, Wang J, Xu X, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020;12:4594–4603. PMID: 32913533
- Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. 2021;36:474–481. PMID: 32677707Shioji N, Everett T, Suzuki Y, Aoyama K. Pediatric sedation using dexmedetomidine and remimazolam for magnetic resonance imaging. J Anesth. 2022;36:1–4.