Anesthesiology News
Advocate Illinois Masonic Medical Center
Clinical Professor of Anesthesiology and Surgery
Departments of Anesthesiology and Surgery
University of Illinois at Chicago
Chicago, Illinois
Advocate Illinois Masonic Medical Center
Chicago, Illinois
There has been growing interest among practitioners of regional anesthesia concerning its provision to people with a preexisting neurologic condition and, specifically, a history of neuropathy.
The benefits of providing regional anesthesia for patients undergoing a variety of surgical interventions have been well established. In 1999, Alon P. Winnie, MD, a pioneer of regional anesthesia and co-founder of the American Society of Regional Anesthesia and Pain Medicine (ASRA) in 1975, outlined 8 major advantages of regional blocks in Pain Management and Regional Anesthesia in Trauma, which continue to be recognized and increasingly appreciated today.1 The advantages of regional blocks, according to Dr Winnie, compared with use of opioids or central neuraxial blocks, include the following:
- superior postoperative analgesia;
- improved rehabilitation efforts;
- decreased perioperative nausea and vomiting;
- faster emergence and recovery;
- earlier mobilization (unilateral block);
- unilateral, postganglionic sympathetic block with less associated trespass;
- extended benefits via continuous catheters; and
- faster outpatient discharge.1
Neuropathy is defined as “deranged function and structure of a peripheral motor, sensory, or autonomic nerve, involving the entire nerve or selected levels.”2 Neuropathies may be either acquired or inherited and encompass several distinct entities commonly encountered in routine clinical practice affecting either the central nervous system (CNS) or peripheral nervous system (PNS) (Table 1).
| Table 1. Types of Neuropathies Involving the CNS and PNS |
| Central Pain Syndromes |
|---|
|
| Peripheral Pain Syndromes |
|
| CNS, central nervous system; CMT, Charcot-Marie-Tooth; CTS, carpal tunnel syndrome; HNPP, hereditary neuropathy with liability to pressure palsy; GBS, Guillain-Barré syndrome; PNS, peripheral nervous system; UNS, ulnar nerve syndrome |
Peripheral neuropathy exists in 4 cardinal patterns:
- polyneuropathy (generalized disorder);
- mononeuropathy (disease involving a single nerve);
- mononeuritis multiplex (inflammation of several separate nerves in unrelated parts of the body); and
- autonomic neuropathy (collection of syndromes and diseases affecting the autonomic neurons, either parasympathetic or sympathetic, or both).
A review of major reference works dedicated to regional anesthesia spanning 96 years contains only a few pages in which the issue of central neuraxial anesthesia or peripheral nerve block (PNB) was discussed in the context of neuropathy.3-10 In the 1953 book Regional Block, Moore stated: “Whenever preexisting neurological disorders are present … the possibility of a medicolegal suit should be evaluated before administering a spinal, caudal, epidural or nerve block.”4 In the 1978 text Epidural Analgesia, Bromage opined, “Any postoperative neurological complications arising after regional anesthesia are likely to be attributed to the anesthetic. The nerve-blocking effects of subarachnoid and epidural anesthesia are so dramatic that it is perhaps natural to propose an etiology based on shallow assumptions of cause and effect.”5 Furthermore, Bromage wrote, “Although it is difficult to see how an epidural block could have an adverse effect on these conditions [preexisting neurologic diseases] the anesthesiologist will avoid the possibility of becoming involved in a post hoc, ergo propter hoc [meaning ‘after this, therefore because of this’] litigation claim should a natural exacerbation of the disease develop after the operation.”5
In 1999, Finucane, in Complications of Regional Anesthesia, noted, “This dearth of information makes it impossible to define specific guidelines for the use of regional anesthesia in the patient with neuromuscular disease. It is also clear that in many neurologic and neuromuscular disorders, there may be a distinct advantage to the use of regional anesthesia over general anesthesia.”6 In the second edition of his book, published in 2007, Finucane stated, “However, a study of significant size to confirm or support the safety of regional anesthesia in these patients continues to remain scarce.”7
In 2007, Neal and Rathmell, in Complications in Regional Anesthesia & Pain Medicine, stated, “However, it has also been suggested that patients with preexisting neurologic deficits may be at increased risk as well. … The presence of chronic underlying neural compromise secondary to mechanical, ischemic, toxic or metabolic derangements may place these patients at increased risk.”8
These authors were among the first to recognize the importance of the “double-crush phenomenon” in defining the etiology of several of these neurologic insults that occur after a regional anesthetic procedure in compromised neural states. According to the postulated theory, a nerve with a mild preexisting neuronal injury at 2 separate sites may cause distal denervation (ie, double-crush), or an axon with a diffuse preexisting underlying disease process (toxic, metabolic, ischemic) and potentially impaired axonal flow throughout the neuron (symptomatic/asymptomatic) may predispose the axon to distal denervation after a single minor neural insult at site X (ie, double-crush).
Finally, in 2009, in Cousins and Bridenbaugh’s Neural Blockade in Clinical Anesthesia and Pain Medicine, Cousins et al stated, “The most conservative legal approach is to avoid regional anesthesia in these [preexisting neurologic-disordered] patients. … The decision to proceed with regional anesthesia in these [high-risk] patients should be made on a case-by-case basis.”10
The controversy regarding the benefits provided by regional anesthesia and likelihood of incurring undue risks by exacerbating the pathologic state in patients with preexisting neurologic processes continues. Some reports exist documenting worsening of subclinical or overt neuropathy after regional anesthesia, which shine an unfavorable light on peripheral nerves and neuraxial blocks and potentially hinder administration of these anesthetic approaches, even in cases in which likely benefits far outweigh the risks. The uncertainty experienced by many practitioners is compounded by a paucity of scientific evidence on the topic, which mainly consists of anecdotal reports, a lack of robust scientific cause-and-effect evidence, and unavailability of guidelines on safe practices in this vulnerable patient population. The goal of this discussion is to highlight the current state of knowledge regarding the application of PNBs and neuraxial anesthesia in patients with specific preexisting neuropathies, and the influence, or lack thereof, of the anesthetic technique selected on the neuropathic syndrome.
Approach to the Patient With a Preexisting Neuropathy
The approach to the patient with a preexisting neuropathy presenting to the operating room as a potential candidate for regional anesthesia entails performing the standard preoperative evaluation and documentation for every patient. However, a special emphasis on the neurologic examination and exercise tolerance is required. These patients may require a longer preoperative evaluation and may especially benefit from a visit to the preanesthesia clinic. It is imperative to document baseline neurologic function and sensory and motor deficits, as well as clearly mark the affected areas. Additionally, previous medical records, neurologic consultations, imaging studies, and other relevant workup and management of the preexisting neurologic condition should be reviewed. Moreover, the respiratory and cardiovascular systems may be harbingers of a systemic neurologic disorder, and need to be evaluated especially carefully in patients manifesting a peripheral neuropathic process. It is crucial to evaluate volume status, beat-to-beat heart rate variability, resting tachycardia, orthostatic hypotension, cardiac dysrhythmias, and the presence of impotence.
If regional anesthesia is determined to be a prudent choice for these individuals, a comprehensive discussion detailing the relevant risks, benefits, and alternatives should be undertaken and documented. This requires familiarity with scientific evidence regarding the risks for potential complications in patients with preexisting neuropathies compared with their unaffected counterparts. In fact, according to Brull et al, most academic anesthesiologists specializing in regional anesthesia are unable to provide patients with the actual substantive risks, in most cases, from regional block in non-neurologically impaired individuals.11 This phenomenon is not unique to academic regional anesthesiology experts but also extends, as expected, to a population of ASRA members.12 In a survey of 3,732 ASRA members, with 801 (21.7%) responding, the likelihood of disclosing the pertinent risks associated with regional block to patients was inconsistent, implying that—under ideal circumstances—most anesthesiologists either are not cognizant of the relevant risks or do not always discuss these risks with all patients.
What is the likelihood of developing new neuropathic pain in neurologically compromised patients undergoing a regional block? The evidence is still inadequate to determine the answer to this question with any degree of certainty. However, predicting the incidence in a nonimpaired patient is possible, and may be used as the best-case scenario in this patient population. In a 2007 publication, Brull et al reviewed 10 years’ worth of data from 32 studies that met the inclusion criteria and were designed to measure neuropathy rates in patients undergoing neuraxial blocks and PNBs.13 Although the incidence of perioperative neuropathy was generally 100 times higher after PNBs than after neuraxial blocks, the likelihood of complete resolution without long-term sequelae was much higher after neuropathy induced by PNBs than by central neuraxial blocks (Table 2).13
| Table 2. Neurologic Complications After Regional Anesthesia | |
| Type of Anesthetic Block | Relative Incidence |
|---|---|
| Central Neuraxial Blocks | |
| Spinal anesthesia | 3.78/10,000 (0.04%) |
| Epidural anesthesia | 2.19/10,000 (0.02%) |
| Peripheral Nerve Blocks | |
| Interscalene brachial plexus block | 2.84/100 (2.84%) |
| Axillary brachial plexus block | 1.48/100 (1.48%) |
| Femoral nerve block | 0.34/100 (0.34%) |
| Based on reference 13. | |
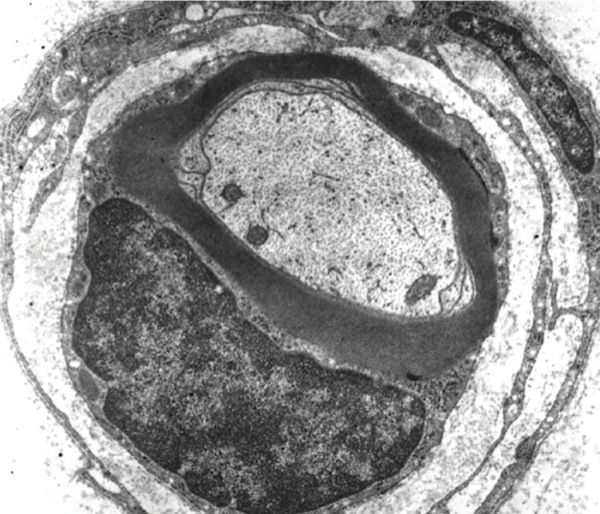
Another question without definitive answer is why certain nerves are susceptible to sustaining a neuropathy after regional anesthesia. Hogan outlined some of the characteristic features of nerves that predispose them to suffering insults that might be long term or permanent.14 Nerves are not solid unyielding structures but rather contain a matrix arrangement of a multitude of neural elements that defy ready characterization, due to the almost random location of axons in the matrix (Figures 1 and 2). Hogan expressed that the toxicity of injected anesthetic solutions used for regional anesthesia is proportional to the duration of the nerve’s exposure. In addition, agent-specific alterations in peripheral blood flow can cause ischemic changes in nerves. For example, epinephrine alone produces vasoconstriction, but this does not directly equate with nerve injury. Mechanical effects of nerve blocks, including nerve edema and endoneurial herniation, may contribute to nerve injury. An ischemic insult to the nerve initially results in depolarization, followed by an increase in spontaneous neural activity. Finally, after less than 2 hours of ischemic time, nerve function typically returns to normal within about 6 hours.14Neurologic injury may be related to neurotoxicity, mechanical trauma, or neural ischemia of local anesthetics.

Multiple risk factors predisposing to neurologic complications after neuraxial anesthesia and PNBs have been investigated. The emergence of postprocedural neurologic sequelae is influenced by patient-, surgery-, and anesthesia-related risk factors (Table 3).15,16 Of note, many individuals undergoing surgery with regional anesthesia may have subclinical neuropathies. These patients may be sensitive to the nerve-blocking effects of local anesthetics, and may respond to decreased concentrations of these agents. The effect of extended-duration liposomal bupivacaine suspensions on nerve function in neuropathic pain conditions has not been elucidated.
| Table 3. Factors Contributing to Neuraxial and Peripheral Nerve Injury |
| Neuraxial Injury |
|---|
| Mechanical damage |
|
| Neural ischemia |
|
| Local anesthetic toxicity |
|
| Peripheral Nerve Injury |
| Patient-related factors |
|
| Surgery-related factors |
|
| Anesthetic-related factors |
|
| DM, diabetes mellitus; GA, general anesthesia; GI, gastrointestinal; HTN, hypertension; MAC, monitored anesthesia care Based on references 15 and 16. |
Regional Anesthesia and Conditions Of Preexisting Neuropathy
Central Pain Syndromes
Multiple Sclerosis
Multiple sclerosis (MS) is a commonly occurring inflammatory degenerative disease of poorly defined etiology, characterized by demyelination lesions in the brain and spinal cord. It follows a progressive-remitting course, and is associated with sensory and motor deficits, vision changes, autonomic dysfunction, and bowel and urinary issues. Hemodynamic, temperature, or metabolic perturbations during the perioperative period may worsen symptoms postoperatively.17 Patients with MS may have subclinical or overt neurologic deficiencies. Neurologic symptoms may worsen postoperatively due to any of the abovementioned stressors regardless of the anesthetic technique employed.18 Historically, PNBs have been considered safe given the presumed lack of PNS involvement. Safety concerns related to PNBs in patients with MS emerged after evidence documenting peripheral demyelinating lesions became available.19,20 Additionally, brachial plexopathy was reported after interscalene brachial plexus block in a patient with possible “silent” peripheral demyelination consistent with MS,21 whereas prolonged duration of the blockade was described after techniques proximal to the central neuraxis, such as a paravertebral block.22
The safety of neuraxial anesthesia in MS is being actively investigated, but the available literature offers little guidance for clinical decision making. Several case reports document deterioration of neurologic symptoms in patients with MS or a new diagnosis of the disease made after neuraxial anesthesia.23-26 However, independent prospective cohort studies have shown successful use of neuraxial anesthesia in obstetric patients with MS without triggering relapse or worsening of the neurologic deficits.27,28 A 2017 systematic review of 37 publications (11 studies and case series; 26 case reports) discussing the use of neuraxial anesthesia (231 patients; 243 interventions) concluded that the available literature does not support the claim that neuraxial anesthesia results in a worsened clinical course for MS, and therefore remains a viable option for patients suffering from MS.29 Of note, the risks are not equal for the 2 central neuraxial techniques; epidural anesthesia is recommended as the preferred anesthetic method; it has a higher safety profile than a subarachnoid block.30 It has been found that spinal anesthesia may exacerbate neurologic dysfunction in patients with MS, likely due to increased sensitivity of the demyelinated spinal cord areas to local anesthetics deposited in the intrathecal space,23,31 or by blocking sodium channels in demyelinated regions of the CNS.32
Spinal Stenosis and Compressive Radiculopathy
Age-related degenerative changes of the spine result in stenosis or narrowing of the spinal canal or neural foramina, leading to compression of spinal nerve root(s) and emergence of radiculopathy in the limb(s). The presence of spinal stenosis or compressive radiculopathy, but not a history of spine surgery, has been shown to increase the risk for secondary neurologic injury by 1.1% in a retrospective study of 937 patients who underwent neuraxial anesthesia for surgical procedures compared with the general population.33 Although prior spine surgery is not considered a risk factor for neurologic complications, “The Second ASRA Practice Advisory” from 2015 recommends obtaining radiological or fluoroscopic imaging before administering neuraxial anesthesia in this patient population.18 The probable reason for neurologic complications developing in this group of patients is the narrowing of the spinal canal leading to a buildup of pressure within the canal and around the neural structures after injection of anesthetic solution or introduction of an epidural catheter.34
Peripheral Pain Syndromes
Diabetic Polyneuropathy
Distal symmetric sensorimotor polyneuropathy is the most frequently encountered neuropathy affecting diabetic patients and is among the most common neuropathic pain conditions known. The known microangiopathy caused by the disease is characterized by decreased blood flow and resultant ischemic hypoxia, and may result in an increased sensitivity to local anesthetic medications. Patients undergoing neuraxial techniques may be at the highest risk for adverse events, according to a retrospective study by Hebl et al of 567 patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy who underwent neuraxial analgesia or anesthesia.35 Two patients (0.4%) developed a new or progressive deficit after an otherwise uneventful neuraxial block.35 The incidence of new neuropathy after spinal block (0.3%) was approximately 9 times higher than that reported by Brull et al for neuropathy developing after spinal blocks in noncompromised patients, and approximately 25 times higher for epidural blocks (0.5% vs 0.02%).13,35
The rate of neuropathy in patients with diabetes receiving PNBs remains unknown but must be considered on the basis of observations found in isolated case reports, such as that of Horlocker et al, in which the same patient undergoing continuous blocks with catheters and infusions developed severe brachial plexopathy on both sides in different settings.36 However, many questions remain unanswered.37 These include whether local anesthetics used in standard doses are more toxic in patients with diabetes than in an unaffected population; whether the dose should be different for patients with diabetes versus those without the disease; whether the use of a peripheral nerve stimulator for nerve localization is less effective in the diabetic population; whether epinephrine doses should be reduced in these patients; and whether information gleaned from animal studies applies to the human condition of diabetes in formulating clinical decisions.37
Although use of peripheral nerve stimulation may be of questionable efficacy for nerve localization in patients with diabetes, some clinicians advocate using ultrasound guidance in these patients as a means of avoiding peripheral nerve stimulation. Sites at al were able to perform popliteal sciatic nerve blockade successfully in 2 different patients for whom use of peripheral nerve stimulation proved unreliable.38 Evoked motor responses were not forthcoming, even with generous stimulating currents of up to 2.4 mA.
Another question is whether the use of continuous techniques predisposes patients with diabetes to persistent neuropathy after surgery. Here, again, the answer must come from retrospective reviews. A review of 405 continuous axillary brachial plexus catheters, including those placed in 40 patients with preexisting neuropathies, found that neither of the 2 new deficits occurred in compromised patients.39 These results imply that prolonged exposure of impaired nerves to infusions of local anesthetics may not necessarily result in a higher risk for postoperative dysfunction.
Some patients with diabetes likely are predisposed to developing new neuropathies after regional blockade. However, the incidence, mechanism, and predictability of this phenomenon remain unclear.
Chemotherapy-Induced Peripheral Neuropathy
The increasing availability of chemotherapeutic agents and better cancer survival rates are related to the growing number of patients suffering from neuropathic pain precipitated by exposure to neurotoxic antineoplastic therapy. Chemotherapy-induced peripheral neuropathy (CIPN) exists in a bilateral “stocking-glove” distribution, and can be either purely sensory or sensorimotor, depending on the offending agent. The reported incidence of CIPN is above 50%.40Although it may occur at any point in the course of chemotherapy, or even afterward, 68.1% of cases develop during the first month of treatment, followed by 60% within the initial 3 months, and 30% at 6 months.41 The degree of neurotoxicity depends on the type of drug, dose, and duration of therapy.42
The risk for developing neurologic complications in patients with CIPN is still unclear, although patients may be susceptible to local anesthetic toxicity, which may worsen preexisting symptoms or uncover subclinical neuropathy.34 A retrospective study of 216 PNBs (165 in the lower extremities; 51 in the upper extremities) performed in 186 patients previously treated with chemotherapy revealed a 2.2% incidence of peripheral nerve injury.43 This number corresponds with the baseline incidence of nerve injury demonstrated by other large-scale studies involving the general population after regional anesthesia for different orthopedic surgeries.13,44-46According to these results, it would appear that regional anesthesia may be safely performed in the presence of CIPN; however, the possibility of sustaining additional neurologic injury should still be considered and caution exercised.
Entrapment Neuropathies
Entrapment neuropathies encompass a wide range of seemingly unrelated conditions that have similar outcomes, such as carpal tunnel syndrome, ulnar neuropathy syndrome, and brachial plexopathy. Entrapment neuropathies may increase susceptibility to the development of neurologic dysfunction after regional block anesthesia, but the data are conflicting to implicate a definitive association. In a retrospective review of 360 patients with ulnar nerve neuropathy undergoing ulnar nerve transposition surgery under general or axillary block anesthesia (72% general; 28% axillary block), 6 patients developed new-onset neural dysfunction.47 Because each had received bupivacaine for the block, the local anesthetic was found to be an independent risk factor for the development of this condition.47 Of note, this 6% incidence of new-onset nerve dysfunction is approximately 4 times greater than the incidence noted in the larger review by Brull et al for axillary brachial plexus block (6% vs 1.48%).13 Perhaps the use of axillary brachial plexus techniques is responsible for the difference, although that appears unlikely. These findings have not been corroborated by an independent investigation. A retrospective review of 1,614 axillary blocks performed on 607 patients, including 31% who had multiple blocks within 1 week (2-10 total blocks), showed that preexisting neuropathy did not increase the risk for neurologic deficits.48
Hereditary Conditions
Several inherited conditions are known to manifest sensorimotor or motor neuropathies, with a wide range of phenotypes of varying severity and clinical presentation. These hereditary neuropathies demand scrutiny when evaluating patients as candidates for regional anesthesia, as they may influence the risk for peripheral nerve injury after blockade. Some examples are hereditary neuropathy with liability to pressure palsy (HNPP) and Charcot-Marie-Tooth (CMT) disease (peroneal muscular atrophy), both of which are discussed in greater detail below; Friedreich’s ataxia; hereditary sensory autonomic neuropathy; Dejerine-Sottas syndrome; Dandy-Walker syndrome; and many other conditions with multisystem involvement and sensorimotor neuropathy.49 Each of these conditions may predispose individuals to postoperative neuropathy after either regional blockade or general anesthesia and should be considered in the differential diagnosis.
Hereditary Neuropathy With Liability to Pressure Palsy
The symptomatology of the disease may not be readily evident preoperatively but may be unmasked after surgical intervention. Therefore the diagnosis of postoperative neurologic dysfunction typically depends on genetic testing to confirm the existence of an inherited disorder. One such condition is HNPP, or tomaculous neuropathy (Figure 3). Genetic testing in such individuals typically demonstrates a 1.5-megabase deletion at chromosome 17p11.2, which encodes the peripheral myelin protein-22 (PMP-22) gene.50 HNPP is a rare autosomal dominant condition with variable penetrance, occurring in 16 per 100,000 people in the general population. The hallmark of the condition is sausage-shaped peripheral nerves, called tomaculas, resulting from abnormal myelin thickness, and painless focal neurologic dysfunction at entrapment sites after minor trauma or compression, which may progress into chronic sensorimotor neuropathy. Patients with HNPP may be prone to the development of schwannomas, which may contribute to the development of pressure palsies, although this association is speculative and has been observed only anecdotally.51 A report of acute brachial plexopathy (lover’s palsy) has been documented in a young man after his partner slept on his arm.52
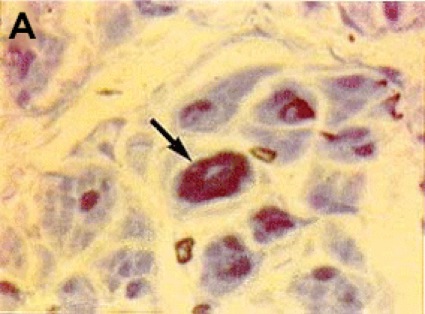
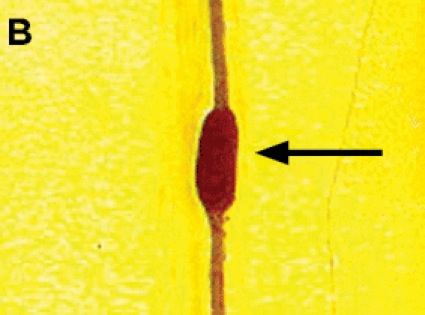
The phenomenon of HNPP is being increasingly recognized and reported. Again, a limited number of case reports form the basis of our appreciation of the role of this entity in the development of neuropathy after surgery and anesthesia. One case report describes the development of intraoperative nerve injury and subsequent diagnosis of HNPP in a woman after breast surgery who underwent general anesthesia.53 Two other case reports discuss safe and successful application of epidural and spinal anesthesia in parturients with HNPP.54,55 While comforting, the evidence is largely insufficient to form any solid conclusions about the superiority of regional anesthesia, in terms of safety and risk for neural injury, over general anesthesia in HNPP patients.
The use of ultrasound guidance for the performance of PNBs may be ideally suited to patients with a documented diagnosis of HNPP. It appears intuitive and inferential that the enlargement (ie, tomacula formation) of peripheral nerves in HNPP may be visualized and thus avoided when advancing needles toward the target nerves, thereby minimizing the potential for traumatic neural injury to occur with the development of neuropathy.56
The possibility of HNPP should be considered in patients who lack obvious risk factors for peripheral nerve injury before the performance of a nerve block or central neuraxial technique, but who present with a postoperative neuropathy subsequent to a regional block.57-62 Overlooking this condition may not only lead to misassignment of blame to the anesthesia care provider but preclude accurate diagnosis, treatment, and prevention of additional injuries with future procedures.
HNPP should become a part of the differential diagnosis especially in obstetrics, where the incidence of postpartum neurologic deficits approximates 1% of parturients; hence the development of a new neurologic deficit should prompt an evaluation and investigation to exclude underlying HNPP.50
CMT Disease
CMT disease is the most common type of hereditary peripheral neuropathy. It is characterized by progressive symmetric muscle weakness and atrophy, depressed tendon reflexes, pes cavus foot deformity, distal sensory neuropathy, and a number of other possible abnormalities. The presentation, disease progression, and morbidity are variable due to significant genotypic and phenotypic heterogeneity. There are 3 types (CMT1 = demyelinating; CMT2 = axonal/nondemyelinating; DI-CMT = dominant intermediate), and 73 affected genes have been elucidated,63 with PMP-22 duplication at chromosome 17p11.2, accounting for 50% of CMT cases.49 The incidence of CMT disease is 1 in 2,500 individuals, and the onset of the disease usually is in childhood or adolescence.64
A small number of case reports and case series indicate that PNBs and central neuraxial techniques may be used safely in patients with CMT disease without precipitating additional neurologic compromise.65-67 CMT disease has been found to be resistant to peripheral nerve stimulation guidance for peripheral nerve localization and, as in patients with diabetes discussed above, may be an ideal indication for the use of ultrasound guidance for nerve localization.65 The possibility of worsening of neurologic symptoms should be considered in patients with CMT disease, and detailed discussion regarding the potential risks and benefits should take place before the provision of regional anesthesia.18
Conclusion
No absolute method exists for predicting how a patient with a preexisting neuropathy will fare with a regional block technique. Whether the neuropathy will remain static or become exacerbated is uncertain. Each case must be evaluated individually, and a full appraisal of the risks and benefits associated with, and the alternatives to, regional anesthesia must be presented to the patient. Documentation of a comprehensive evaluation before the procedure is one of the safest ways to demonstrate that an attempt was made to identify the potential pitfalls in any given situation.
The increasing popularity of ultrasound guidance for nerve and plexus localization, allowing the visualization of target structures, might help clinicians to minimize the likelihood of driving needles into neural structures or unintended sites. Moreover, knowledge of contributing factors in developing neural injury is essential during all anesthetic approaches. Best efforts should be made to reduce the modifiable risk factors to improve the chance for uneventful perioperative events and an optimal recovery. Understanding the relative risks for postprocedural neuropathy in a normal population of patients, and advising patients of such risks, is paramount to reducing the likelihood of misunderstanding and the development of resentment in the case of an unexpected, unwanted result.
References
- Winnie A, Candido K, Torres M. Continuous peripheral nerve blocks for the management of trauma to the extremities. In: Bernstein RL, Rosenberg AD, Grande CM, eds. Pain Management and Regional Anesthesia in Trauma. Philadelphia, PA: W.B. Saunders; 1999.
- Adams DA, Victor M. Principles of Neurology. 5th ed. New York, NY: McGraw-Hill; 1993.
- Labat G. Regional Anesthesia: Its Technic and Clinical Application. 1st ed. Philadelphia, PA: WB Saunders; 1922.
- Moore DC. Regional Block. 4th ed. Springfield, IL: Charles C. Thomas; 1953.
- Bromage PR. Epidural Analgesia. Philadelphia, PA: WB Saunders; 1978.
- Finucane BT. Complications of Regional Anesthesia. 1st ed. New York, NY: Churchill-Livingstone; 1999.
- Finucane BT. Complications of Regional Anesthesia. 2nd ed. New York, NY: Springer; 2007.
- Neal JM, Rathmell JP. Complications in Regional Anesthesia & Pain Medicine. Philadelphia, PA: Saunders Elsevier; 2007.
- Hadzic A. Textbook of Regional Anesthesia and Acute Pain Management. New York, NY: McGraw-Hill; 2007.
- Cousins MJ, Carr DB, Horlocker TT, et al. Cousins and Bridenbaugh’s Neural Blockade in Clinical Anesthesia and Pain Medicine. Philadelphia, PA: Lippincott Williams and Wilkins; 2009.
- Brull R, McCartney CJ, Chan VW, et al. Disclosure of risks associated with regional anesthesia: a survey of academic regional anesthesiologists. Reg Anesth Pain Med. 2007;32(1):7-11.
- Brull R, Wijayatilake DS, Perlas A, et al. Practice patterns related to block selection, nerve localization and risk disclosure: a survey of the American Society of Regional Anesthesia and Pain Medicine. Reg Anesth Pain Med. 2008;33(5):395-403.
- Brull R, McCartney CJ, Chan VW, et al. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg. 2007;104(4):965-974.
- Hogan QH. Pathophysiology of peripheral nerve injury during regional anesthesia. Reg Anesth Pain Med. 2008;33(5):435-441.
- Sondekoppam RV, Tsui BC. Factors associated with risk of neurologic complications after peripheral nerve blocks: a systematic review. Anesth Analg. 2017;124(2):645-660.
- Lalkhen AG, Bhatia K. Perioperative peripheral nerve injuries. Cont Educ Anaesth, Crit Care Pain. 2012;12(1):38-42.
- Kuwahira I, Kondo T, Ohta Y, et al. Acute respiratory failure in multiple sclerosis. Chest. 1990;97(1):246-248.
- Neal JM, Barrington MJ, Brull R, et al. The Second ASRA Practice Advisory on Neurologic Complications Associated With Regional Anesthesia and Pain Medicine: Executive Summary 2015. Reg Anesth Pain Med. 2015;40(5):401-430.
- Pogorzelski R, Baniukiewicz E, Drozdowski W. [Subclinical lesions of peripheral nervous system in multiple sclerosis patients]. Neurol Neurochir Pol. 2004;38(4):257-264.
- Misawa S, Kuwabara S, Mori M, et al. Peripheral nerve demyelination in multiple sclerosis. Clin Neurophysiol. 2008;119(8):1829-1833.
- Koff MD, Cohen JA, McIntyre JJ, et al. Severe brachial plexopathy after an ultrasound-guided single-injection nerve block for total shoulder arthroplasty in a patient with multiple sclerosis. Anesthesiology. 2008;108(2):325-328.
- Finucane BT, Terblanche OC. Prolonged duration of anesthesia in a patient with multiple sclerosis following paravertebral block. Can J Anaesth. 2005;52(5):493-497.
- Bader AM, Hunt CO, Datta S, et al. Anesthesia for the obstetric patient with multiple sclerosis. J Clin Anesth. 1988;1(1):21-24.
- Stenuit J, Marchand P. [Sequelae of spinal anesthesia]. Acta Neurol Psychiatr Belg. 1968;68(8):626-635.
- Dalmas AF, Texier C, Ducloy-Bouthors AS, et al. [Obstetrical analgesia and anaesthesia in multiple sclerosis]. Ann Fr Anesth Reanim. 2003;22(10):861-864.
- Fleiss AN. Multiple sclerosis appearing after spinal anesthesia. N Y State J Med. 1949;49(9):1076.
- Pasto L, Portaccio E, Ghezzi A, et al. Epidural analgesia and cesarean delivery in multiple sclerosis post-partum relapses: the Italian cohort study. BMC Neurol. 2012;12:165.
- Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(pt 6):1353-1360.
- Bornemann-Cimenti H, Sivro N, Toft F, et al. [Neuraxial anesthesia in patients with multiple sclerosis – a systematic review]. Rev Bras Anestesiol. 2017;67(4):404-410.
- Pasternak JJ, Lanier WLJ. Diseases affecting the brain. In: Hone RL, Marschall KE, eds. Stoelting’s Anesthesia and Co-Existing Disease. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:248-250.
- Warren TM, Datta S, Ostheimer GW. Lumbar epidural anesthesia in a patient with multiple sclerosis. Anesth Analg. 1982;61(12):1022-1023.
- Sakurai M, Mannen T, Kanazawa I, et al. Lidocaine unmasks silent demyelinative lesions in multiple sclerosis. Neurology. 1992;42(11):2088-2093.
- Hebl JR, Horlocker TT, Kopp SL, et al. Neuraxial blockade in patients with preexisting spinal stenosis, lumbar disk disease, or prior spine surgery: efficacy and neurologic complications. Anesth Analg. 2010;111(6):1511-1519.
- Justiz R, Bautista AF. Regional anesthesia in the patient with preexisting neurological disease. In: Kaye AD, Urman RD, Vadivelu N, eds. Essentials of Regional Anesthesia. 2nd ed. Cham, Switzerland: Springer; 2018:547-548.
- Hebl JR, Kopp SL, Schroeder DR, et al. Neurologic complications after neuraxial anesthesia or analgesia in patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesth Analg. 2006;103(5):1294-1299.
- Horlocker TT, O’Driscoll SW, Dinapoli RP. Recurring brachial plexus neuropathy in a diabetic patient after shoulder surgery and continuous interscalene block. Anesth Analg. 2000;91(3):688-690.
- Williams BA, Murinson BB. Diabetes mellitus and subclinical neuropathy: a call for new paths in peripheral nerve block research. Anesthesiology. 2008;109(3):361-362.
- Sites BD, Gallagher J, Sparks M. Ultrasound-guided popliteal block demonstrates an atypical motor response to nerve stimulation in 2 patients with diabetes mellitus. Reg Anesth Pain Med. 2003;28(5):479-482.
- Bergman BD, Hebl JR, Kent J, et al. Neurologic complications of 405 consecutive continuous axillary catheters. Anesth Analg. 2003;96(1):247-252, table of contents.
- Shah A, Hoffman EM, Mauermann ML, et al. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatry. 2018;89(6):636-641.
- Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461-2470.
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9-17.
- Abcejo AS, Sviggum HP, Mauermann ML, et al. Perioperative nerve injury after peripheral nerve block in patients with previous systemic chemotherapy. Reg Anesth Pain Med. 2016;41(6):685-690.
- Jacob AK, Mantilla CB, Sviggum HP, et al. Perioperative nerve injury after total knee arthroplasty: regional anesthesia risk during a 20-year cohort study. Anesthesiology. 2011;114(2):311-317.
- Jacob AK, Mantilla CB, Sviggum HP, et al. Perioperative nerve injury after total hip arthroplasty: regional anesthesia risk during a 20-year cohort study. Anesthesiology. 2011;115(6):1172-1178.
- Sviggum HP, Jacob AK, Mantilla CB, et al. Perioperative nerve injury after total shoulder arthroplasty: assessment of risk after regional anesthesia. Reg Anesth Pain Med. 2012;37(5):490-494.
- Hebl JR, Horlocker TT, Sorenson EJ, et al. Regional anesthesia does not increase the risk of postoperative neuropathy in patients undergoing ulnar nerve transposition. Anesth Analg. 2001;93(6):1606-1611, table of contents.
- Horlocker TT, Kufner RP, Bishop AT, et al. The risk of persistent paresthesia is not increased with repeated axillary block. Anesth Analg. 1999;88(2):382-387.
- Bird TD. Charcot-Marie-Tooth (CMT) hereditary neuropathy overview. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: 1993.
- Peters G, Hinds NP. Inherited neuropathy can cause postpartum foot drop. Anesth Analg. 2005;100(2):547-548.
- Heckmann JG, Dutsch M, Buslei R. Hereditary neuropathy with liability to pressure palsy combined with schwannomas of the median and medial plantar nerves. Muscle Nerve. 2007;35(1):122-124.
- Wedderburn S, Pateria P, Panegyres PK. Hereditary neuropathy with liability to pressure palsy presenting as an acute brachial plexopathy: a lover’s palsy. Case Rep Neurol. 2014;6(3):281-286.
- Wijayasiri L, Batas D, Quiney N. Hereditary neuropathy with liability to pressure palsies and anaesthesia: peri-operative nerve injury. Anaesthesia. 2006;61(10):1004-1006.
- Lepski GR, Alderson JD. Epidural analgesia in labour for a patient with hereditary neuropathy with liability to pressure palsy. Int J Obstet Anesth. 2001;10(3):198-201.
- Berdai S, Benhamou D, Equipe S-A. [Regional anaesthesia for labor adn delivery in a parturient with neuropathy with liability to pressure palsy (tomaculous neuropathy)]. Ann Fr Anesth Reanim. 2004;23(10):1011-1014.
- Beekman R, Visser LH. Sonographic detection of diffuse peripheral nerve enlargement in hereditary neuropathy with liability to pressure palsies. J Clin Ultrasound. 2002;30(7):433-436.
- Stogbauer F, Young P, Kerschensteiner M, et al. Recurrent brachial plexus palsies as the only clinical expression of hereditary neuropathy with liability to pressure palsies associated with a de novo deletion of the peripheral myelin protein-22 gene. Muscle Nerve. 1998;21(9):1199-1201.
- Lane JE, Foulkes GD, Hope TD, et al. Hereditary neuropathy with liability to pressure palsies mimicking multifocal compression neuropathy. J Hand Surg Am. 2001;26(4):670-674.
- Koc F, Guzel R, Benlidayi IC, et al. A rare genetic disorder in the differential diagnosis of the entrapment neuropathies: hereditary neuropathy with liability to pressure palsies. J Clin Rheumatol. 2006;12(2):78-82.
- van de Wetering RA, Gabreels-Festen AA, Timmerman V, et al. Hereditary neuropathy with liability to pressure palsies with a small deletion interrupting the PMP22 gene. Neuromuscul Disord. 2002;12(7-8):651-655.
- Muglia M, Patitucci A, Rizzi R, et al. A novel point mutation in PMP22 gene in an Italian family with hereditary neuropathy with liability to pressure palsies. J Neurol Sci. 2007;263(1-2):194-197.
- Sander MD, Abbasi D, Ferguson AL, et al. The prevalence of hereditary neuropathy with liability to pressure palsies in patients with multiple surgically treated entrapment neuropathies. J Hand Surg Am. 2005;30(6):1236-1241.
- Stojkovic T. Hereditary neuropathies: an update. Rev Neurol (Paris). 2016;172(12):775-778.
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet. 1974;6(2):98-118.
- Dhir S, Balasubramanian S, Ross D. Ultrasound-guided peripheral regional blockade in patients with Charcot-Marie-Tooth disease: a review of three cases. Can J Anaesth. 2008;55(8):515-520.
- Bui AH, Marco AP. Peripheral nerve blockade in a patient with Charcot-Marie-Tooth disease. Can J Anaesth. 2008;55(10):718-719.
- Tanaka S, Tsuchida H, Namiki A. [Epidural anesthesia for a patient with Charcot-Marie-Tooth disease, mitral valve prolapse syndrome and IInd degree AV block]. Masui. 1994;43(6):931-933.


Leave a Reply
You must be logged in to post a comment.