AUTHORS: Luke S. Janik, MD et al
Anesthesia Patient Safety Foundation Volume 37, No. 3 • October 2022
| Reprinted from Anesthesia & Analgesia, June 2022 • Volume 134 • Number 6, pages 1192–1200, with permission from International Anesthesia Research Society. Professional titles and nomenclature were standardized and modified within the text consistent with APSF policy. |
In this Pro-Con commentary article, the authors have been asked to refute or support a position regarding anesthesia for endoscopic retrograde cholangiopancreatography (ERCP). ERCPs are unique in that they not only necessitate a shared airway but are typically performed in the prone (or semiprone) position on a special procedural table. Moreover, procedural times can vary from <1 hour to several hours.
The practice of medicine often varies among medical professionals when a defined standard of care does not exist. The cause of this variability is multifactorial. Patient factors and comorbidities, practitioner skills and experience, procedural needs, and the absence of data are a few of the considerations. Thus, it is not surprising that the primary mode of anesthesia for gastrointestinal (GI) endoscopy patients is sharply partitioned between those advocating for monitored anesthesia care (MAC) versus those who rely on general endotracheal anesthesia (GEA).
The importance of this debate is even more relevant because of the increasing recognition of significant potential morbidity and mortality associated with these anesthetics and procedures. A Closed Claims report from the American Society of Anesthesiologists (ASA) suggests that adverse events in nonoperating room anesthesia (NORA) sites result in a higher incidence of severe complications—including death and permanent brain damage—than similar events occurring in the operating room.1 Indeed, the GI suite accounted for the highest percentage of adverse events across all NORA locations.
Anesthesia professionals will certainly encounter an increasing demand for services in the NORA setting and, especially, the GI suite. Thus, this Pro-Con debate provides insights into the care plan decision of MAC versus GEA for ERCP procedures, as summarized in Table 1. Our patients will ultimately benefit from further systematic clinical study of these variable approaches and their associated outcomes.
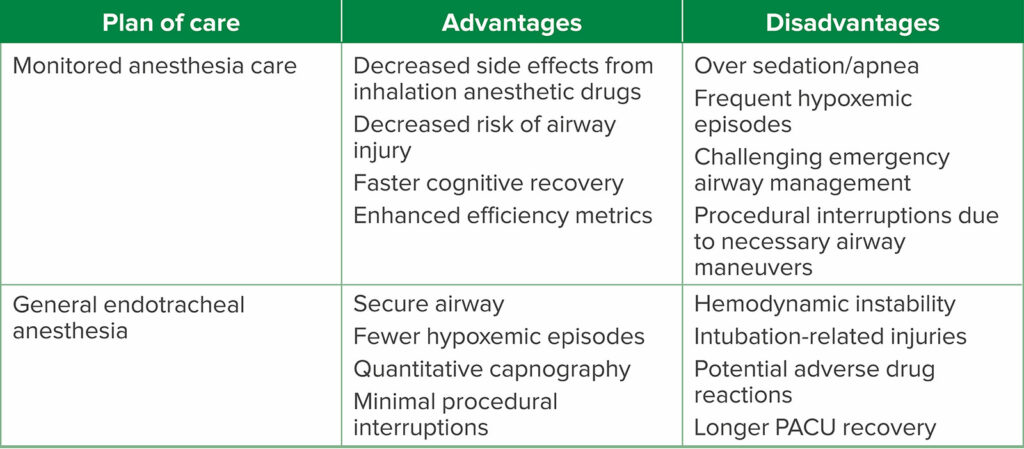
Table 1: Pro-Con Debate Summary.
Abbreviations: ERCP, endoscopic retrograde cholangiopancreatography; GEA, general endotracheal anesthesia; MAC, monitored anesthesia care; NORA, nonoperating room anesthesia; SRAE, sedation-related adverse event.
PRO: ANESTHESIA FOR ERCP IS BEST DONE WITH MAC
Samantha Stamper, MD, and
Christopher A. Troianos, MD, FASE, FASA
ERCP utilizes fluoroscopy and endoscopy for both diagnostic and therapeutic interventions. Its use facilitates the evaluation of the liver, gallbladder, bile ducts, and pancreas. In recent years, ERCP has been predominantly used for therapeutic interventions given the advent of advanced endoscopy therapeutic techniques and imaging technology (eg, magnetic resonance imaging with magnetic resonance cholangiopancreatography, endoscopic ultrasound).13 Such interventions include biliary sphincterotomy, gallstone extraction or fragmentation, biliary and pancreatic duct stenting, and pancreatic pseudocyst drainage.12,13 Many of these procedures previously required open or laparoscopic surgery for treatment, but ERCP is now a viable, cost-effective, and preferable alternative.
Advanced endoscopic interventions have the added benefit of being minimally invasive, less painful, and seldom require muscle paralysis.6 More than 500,000 ERCPs are performed annually in the United States, with a majority requiring anesthesia services.14 ERCPs are more often performed in older patients; many of whom have a greater burden of comorbid conditions.13 While there is currently no outcome evidence performed based on prospectively randomized trials as to whether MAC or GEA is superior for patients undergoing advanced endoscopy interventions, there is convincing clinical rationale to prioritize a “MAC-first” approach in the majority of these endoscopy patients. While anesthetic plans are always tailored to each specific individual, the experienced endoscopy team will recognize that the MAC approach may be the superior one, particularly for healthier patients with a normal or near-normal body mass index (BMI). Clear communication between the endoscopist and anesthesia professional is critical. For instance, the specific indication for the ERCP (therapeutic versus diagnostic) and case duration are vital to create a shared mental model and will likely contribute to the determination of the optimal anesthetic. For example, if the intervention plan is a straightforward removal of a biliary stent, then MAC may be most appropriate. By contrast, drainage of a complex, septated pancreatic pseudocyst with necrotic walls will almost certainly require GEA. Therefore, the time and invasiveness of the intervention are vital inputs to anesthetic choice, and the advantages and disadvantages of each anesthetic technique must be considered (Table 2).
Table 2: Advantages and Disadvantages of Each Anesthetic Plan of Care.
Specific facility factors similarly contribute to the choice of the optimal anesthetic. These considerations include proximity to the main operating rooms, readiness of rescue equipment, adequate post anesthesia care unit, and the availability of additional help, if needed. Other considerations include the physical footprint of the anesthesia workspace, which is often limited due to specialized equipment (eg, endoscopy supplies, radiographic imaging equipment, ancillary display/viewing towers). Communication with both the institution and endoscopy team before the procedure is important to help mitigate any untoward complications. Moreover, the prudent practitioner must always ensure a clear plan and pathway are in place in case emergent airway rescue is needed. The factors listed above may contribute to the decision to prioritize MAC.
Complex endoscopy—particularly ERCP procedures— are routinely performed in the prone or semiprone position, which can limit ready access to the airway and/or impact venous return and cardiovascular stability.2 However, this position usually maintains pulmonary blood flow and ventilation distribution (V/Q match) in the lungs, especially in the nonintubated (e.g., MAC) patient. Furthermore, the endoscope itself can mitigate airway collapse by acting as a stent.15 Prone position has multiple additional positive effects on respiratory function, specifically increasing functional residual capacity (FRC) and the arterial Po2.2
A major concern regarding MAC in the prone position is the potential need for urgent or emergent access to the airway, with the potential need for emergent endotracheal intubation. One potential, provocative strategy is for an adequately trained endoscopist to perform a gastroscope-facilitated endotracheal intubation. This requires a smaller endoscope capable of being introduced into the trachea and an endoscopist who possesses these skills, readily facilitated by an anesthesia professional. The “ultraslim” gastroscope functions similarly to a bronchoscope and has an outer diameter of 5.4 mm that can accommodate an adult endotracheal tube over the scope.16 In a review of over 3400 patients undergoing ERCP (46% with GEA versus 54% with MAC), the overall conversion rate from MAC to GEA was low at 2.3%. The authors described their successful use of gastroscope-facilitated tracheal intubation in 16 patients due to retained food in the stomach and/or hypoxia.17 An additional benefit of the gastroscope is that aspirated material can be immediately suctioned from the trachea and bronchi, thereby decreasing the risk of respiratory complications.17 Extubation was successful in all patients who underwent gastroscope-facilitated intubation, and no patients had radiographic evidence of aspiration pneumonia.17
This novel approach to rescue the compromised or failing airway obviates the most commonly identified concern by clinicians considering the use of MAC in the prone or semiprone position. The endoscopist in the above-mentioned study was self-trained in this technique, highlighting the fact that there is currently no formal training or credentialing process for gastroscope-facilitated intubation.17 This technique should only be considered under the direct supervision of an anesthesia professional or performed by an anesthesia professional. One important caveat to using the ultraslim gastroscope for intubation is that the endoscopist must switch from the traditional side-viewing ERCP gastroscope to the ultraslim gastroscope loaded with an endotracheal tube. This exchange of gastroscopes provides the benefit of suctioning the stomach, esophagus, and hypopharynx on withdrawal—immediately before intubation—but should be performed in an expedited fashion to minimize potential delay to intubation.
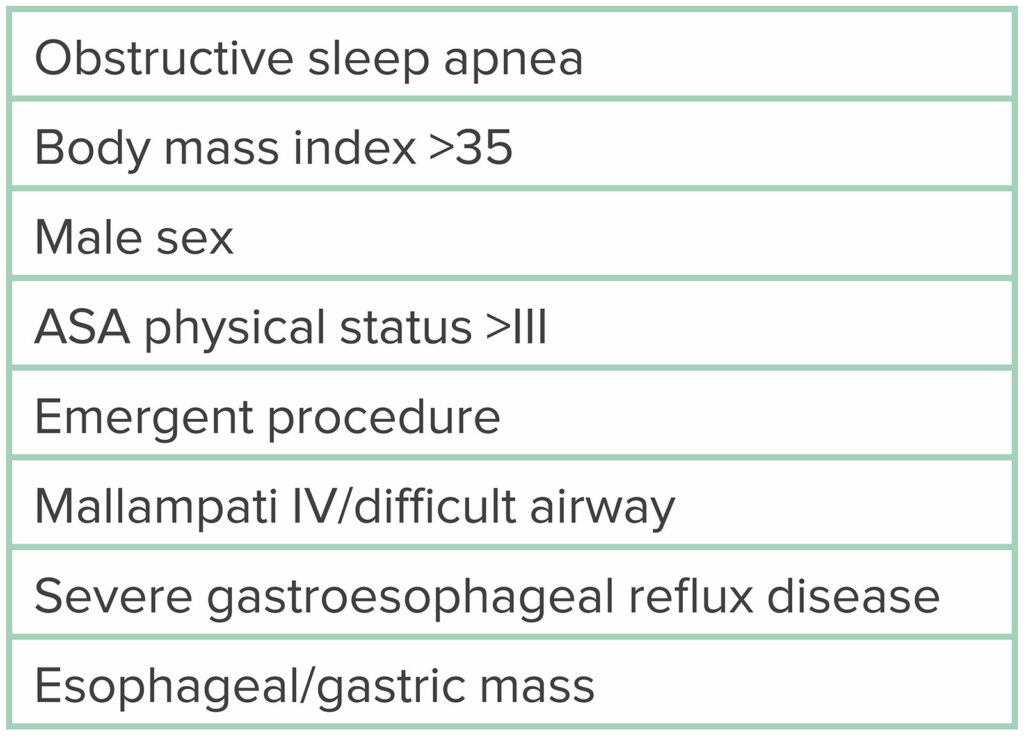
Table 3: Risk Factors for Sedation-Related Adverse Events During MAC.
Before proceeding with MAC for ERCP, risk factors for sedation-related adverse events (SRAEs) must be considered, as highlighted in Table 3. Conditions that increase the likelihood of aspiration are considered by many to be risk factors for SRAEs. Numerous studies have shown MAC to be a safe option for ERCP, especially in patients with minimal risk factors for SRAEs. A large, decade-long, population-based study at multiple endoscopy centers in the United States found no significant difference in overall serious adverse events between ERCPs performed with MAC (n = 8395) versus GEA (n = 10,715; odds ratio [OR] = 1.04, 95% confidence interval [CI], 0.76–1.43).2,3 Albeit, the majority of these patients were relatively healthy (ASA physical status I and II), and the authors did not attempt to control for selection bias. There was no significant difference in adverse events between ASA physical status I and ASA physical status II patients (OR = 0.84 [0.49–1.46]), nor was there a difference between ASA physical status III and ASA physical status II patients (OR = 1.30 [1.00–1.69]). In fact, the data suggest that only ASA physical status IV patients were noted to have a significantly higher risk of adverse events with MAC (OR = 3.19 [2.00–5.09]).2,3 In another prospective observational study, the decision of MAC or GEA was left to the anesthesia professional, with 393 patients receiving MAC and 45 patients receiving GEA.4 The conversion rate of MAC to GEA was 3.7%. Notably, 25% of the patients converted to GEA were ASA physical status IV patients.2,4 Given the inherent selection bias of this study, it comes as no surprise that the mean BMI was higher in the GEA than the MAC group, as was the percentage of ASA physical status IV patients.4,6 Nonetheless, adverse event rates between MAC and GEA were not statistically different, and the study authors concluded that MAC is feasible and well tolerated for healthier, nonobese patients who are evaluated before the procedure by an anesthesia professional.2,4,6
Clinical monitoring during MAC for ERCP should follow routine standards for basic anesthesia monitoring, which involves continually evaluating a patient’s oxygenation, ventilation, circulation, and temperature18; this includes measuring noninvasive blood pressures, pulse oximetry, electrocardiography, and capnography. Many of the airway devices (eg, nasal cannulas or simple facemask) used in MAC are capable of monitoring end-tidal CO2 and detect apnea well before the onset of hypoxia.4,19 Additional monitoring modalities are available for detecting apnea before the decrease in pulse oximetry, including impedance pneumography and—less commonly used in the operating room setting—an acoustic respiration rate monitor.
All MAC anesthetics begin with adequate preoxygenation. This is crucial in preventing hypoxemia—an obvious precursor to more serious adverse events (eg, cardiac arrhythmias, hypotension, and cardiac arrest).20 Ideally, preoxygenating for 3 minutes or 4 vital capacity breaths can provide at least 4 minutes of “safety time” before a patient begins to desaturate without adequate ventilation.21 Adequate preoxygenation in obese patients is of the utmost importance despite the reduction in “safety time” given the decreased FRC. It is important to keep in mind that obese patients often have concomitant pulmonary and systemic comorbidities that may be further exacerbated while in the prone position despite preoxygenation. Appropriate preoxygenation before the administration of sedation increases the margin of safety should transient apnea/hypoventilation occur with the initial bolus dose of propofol. In these instances, preoxygenation allows the anesthesia and endoscopy team more time to intervene with corrective measures (eg, jaw thrust and endoscope insertion for stimulation) before the onset of hypoxemia.
There are several ways to provide supplemental oxygen to patients undergoing ERCP with MAC, including low- to high-flow nasal cannulae, procedural oxygen masks, and specialized endoscopy masks. These airway devices all vary based on the amount of fractional inspired oxygen that can be delivered. Many of these devices are also capable of providing capnography monitoring during the procedure. Before the initiation of sedation, many centers will also have the patient place a bite block into their mouth to prevent biting the endoscope. Many bite blocks have a built-in airway feature or even a suction port that can help clear airway secretions.15 In addition to ensuring the airway delivery device is comfortable, having the patient self-position can help decrease the risk of compression or nerve injury that might otherwise be unrecognized in a patient undergoing GEA. An added benefit to self-positioning is that fewer staff are required to assist with transferring the patient as would be needed if the patient was under general anesthesia.
There are numerous additional supplements to consider during MAC for advanced endoscopic procedures. Premedication with glycopyrrolate reduces secretions and improves the efficacy of topical anesthetics.22 In fast turnover endoscopy centers, this would need to be administered in the preoperative area to take effect before the procedure. Patients should be counseled about the side effects of each medication accordingly. Before initiating sedation, topical pharyngeal anesthesia blunts the stimulation from scope insertion. Options for topicalization include local anesthetic sprays, which usually contain benzocaine or lidocaine as the active ingredient, or viscous lidocaine, which the patient can swish around their mouth and subsequently swallow. If using benzocaine- containing solutions, it is important to use caution due to the risk of methemoglobinemia. The ideal maintenance anesthetic allows for easy titration, rapid recovery, and minimal side effects while maintaining spontaneous ventilation. Propofol is easily titrated to maintain spontaneous ventilation while simultaneously providing moderate to deep sedation.23 If analgesia is needed, adding a shortacting opioid, dexmedetomidine, or ketamine to the intravenous anesthetic is advisable to achieve that goal.22 In addition, endoscopic procedures can be aborted almost immediately by simply removing the scope if urgent access to the airway is required. Scope removal may result in laryngospasm, so one must be ready to urgently treat that potential complication while preparing to secure the airway. Apart from the insertion of the gastroscope, the intensity of stimulation remains relatively constant during ERCP as opposed to the fluctuations that occur during a traditional surgical operation. Due to relatively minimal or absent stimulation, titrating the anesthetic to sustain spontaneous ventilation is usually easily achieved.20 When used alone, propofol sedation allows a return to cognitive baseline within 30 to 45 minutes of discontinuation despite delayed return of psychomotor speed and reaction time.24 Use of MAC avoids the use of both depolarizing and nondepolarizing neuromuscular blocking drugs; many of which have their own unique side effects. There is also less postoperative nausea and vomiting if inhalational anesthetics and opioids are avoided, leading to better patient satisfaction.
GEA is not without risk. Intubation carries the risk of lip, tongue, dental, and eye injuries and, albeit rarely, bronchial rupture or inability to secure an airway and need for a surgical intervention. Succinylcholine is most often used for its rapid onset and short duration, and in the case of endoscopy, paralysis is usually not otherwise necessary. Potential adverse effects of succinylcholine include muscle pain, myoglobinemia, myoglobinuria, and malignant hyperthermia.20 The use of nondepolarizing muscle relaxants is associated with an increased risk of postoperative pulmonary complications from residual neuromuscular blockade.24 The anticholinergic effects associated with reversal of these paralytics must also be considered, though this may be less of an issue at institutions where sugammadex is readily available. The depth of anesthesia required during GEA increases the risk of hypotension, which can subsequently lead to an increased risk of myocardial injury, renal injury, and possibly death.26 Because ERCP is performed in the prone or semiprone position, multiple people are required to safely position and secure the patient while turning from supine to prone position on the fluoroscopy table. There is always a risk of endotracheal tube displacement or accidental extubation during positioning. Finally, the NORA locations often have less support from colleagues and other team members to help during emergencies and anesthesia turnovers, which can subsequently decrease efficiency of the facility. Perbtani et al5 evaluated the impact of GEA on various efficiency metrics in a large interventional endoscopy center. More than 1400 patients who underwent 1635 interventional endoscopic procedures over a 6-month period were analyzed based on time stamps for anesthesia ready time, endoscopist ready time, procedure time, room exit time, time interval between successive procedures, nonprocedural time elapsed, total time elapsed in the endoscopy unit, and number of cases per room per day.2,5 All process efficiency metrics—aside from the time interval between successive procedures—were significantly prolonged among the patients who were intubated compared with nonintubated patients in the interventional endoscopy unit. A secondary aim of the study showed that patients undergoing ERCP were intubated more frequently than those undergoing other procedures (41.3% vs 12.4%).2,5
In conclusion, MAC offers significant benefits over GEA in properly selected patients undergoing ERCP. These benefits include faster cognitive recovery, decreased side effects from the medications used to induce GEA, decreased risk of airway injury, decreased postoperative pulmonary complications, and reduced time spent at the hospital due to quicker induction and shorter time to discharge, thereby enhancing efficiency metrics for the unit, the providers, and the patients. With proper monitoring, supplemental oxygen, and sedation carefully titrated to maintain spontaneous ventilation, MAC during ERCP is a safe and often a superior alternative to GEA.
CON: GEA OFFERS MAJOR ADVANTAGES OVER MAC
Luke S. Janik, MD, and
Jeffery S. Vender, MD, MCCM
ERCP is a frequently performed procedure in the diagnosis and management of pancreaticobiliary disease. Each year, >500,000 ERCP procedures are performed in the United States, with the most common indications being bile duct stones and strictures of the biliary and pancreatic ductal systems.27 ERCP is an invaluable tool in the management of liver, biliary, and pancreatic disease, but is generally considered the most high-risk procedure performed in the GI suite, with an overall procedural complication rate of 4%.28 Procedural complications include pancreatitis (2%–10%), cholangitis/sepsis (0.5%–3%), postsphincterotomy bleeding (0.3%–2%), duodenal perforation (0.08%–0.6%), and death (0.06%).28,29 However, what may be more concerning to those in the anesthesia profession is the high rate of SRAEs during the procedure, with an incidence reported as high as 21%.6,7 This begs the questions of who should be administering anesthesia and monitoring the patient during ERCP and what type of anesthesia should be administered. In this “Pro-Con,” we argue that a qualified anesthesia professional should administer the anesthesia for ERCP, and that GEA offers significant advantages over MAC.
There is wide variability in the delivery models of anesthesia for ERCP. The 3 most common models of anesthesia care delivery are (1) endoscopist-directed sedation (EDS), (2) MAC, and (3) GEA. In the first model, EDS, the intravenous sedation is administered by a member of the GI team—usually a nurse— under the supervision of the endoscopist, who is often simultaneously performing the procedure. The use of traditional “conscious sedation” with titration of benzodiazepines and narcotics has generally fallen out of favor due to high procedure failure rates, poor patient satisfaction, and poor endoscopist satisfaction.30 Consequently, EDS has adopted the use of propofol sedation by nonanesthesia professionals, which the gastroenterology community touts as safe and effective.31–33 In the other 2 models of anesthesia care delivery, the patient is under the care of a qualified anesthesia professional, receiving either MAC with propofol-based sedation or GEA. The choice of anesthesia care delivery model is institution specific and depends on available resources and personnel, procedural complexity, patient characteristics and comorbidities, and individual preferences.
Before we discuss how the anesthesia should be performed, we need to acknowledge where it is performed. The risk of anesthesia in remote locations is widely recognized. An analysis of the ASA Closed Claims database reviewed malpractice claims against anesthesia professionals in remote locations and demonstrated that adverse events in remote locations resulted in higher rates of severe complications—including death and permanent brain damage—than adverse events in the operating room. In fact, the proportion of death was almost double in remote locations versus the operating room (54% vs 29%).11 Respiratory events were more common in remote locations than the operating room (44% vs 20%), with inadequate oxygenation/ventilation identified as the mechanism of injury in 21% of remote location claims versus 3% of operating room claims.11 The closed claims data specific to the GI suite demands further attention. Compared to all other remote venues, the GI suite accounted for the highest percentage of anesthesia malpractice claims (32%), the highest proportion of claims associated with oversedation (58%), and the highest rate of MAC utilization (>80%).11 These data do not come as a surprise to anesthesia professionals. Unfamiliar locations, lack of resources, poor ergonomics, limited assistance, variable cultures of safety, and the physical distance from additional anesthesia equipment and personnel are daily obstacles in the GI suite. In addition, the patients are often older and sicker.11 ERCP introduces other unique challenges, including the routine use of the prone position, limited access to the airway, and the use of an endoscope capable of causing airway obstruction and laryngospasm. Taking all of these challenges into consideration, anesthesia for ERCP carries substantial risk and should be approached with caution.
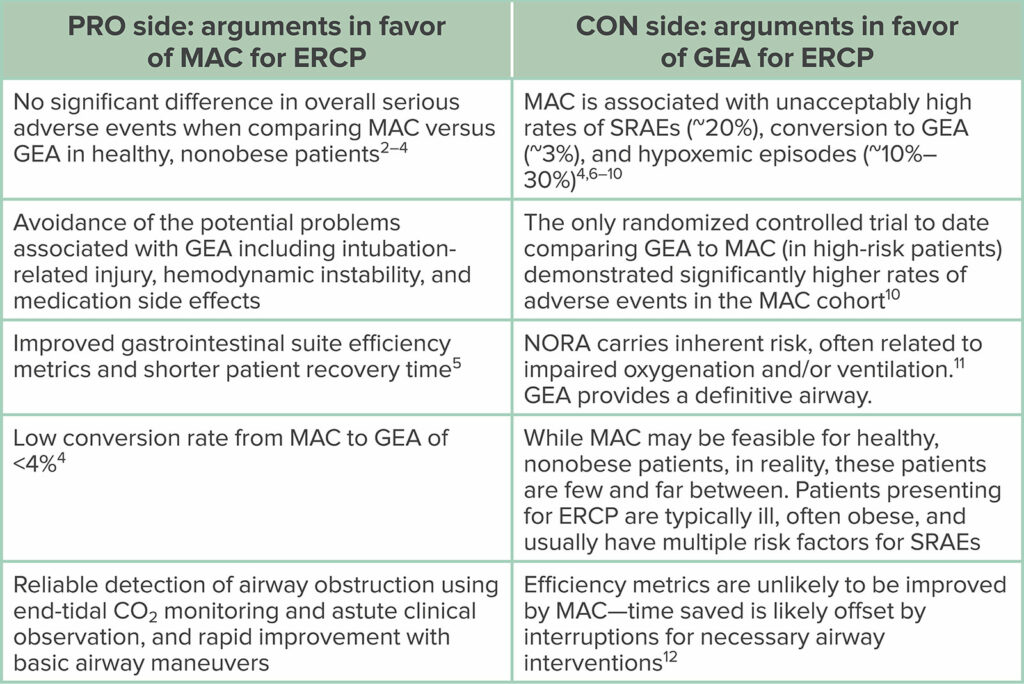
Proponents of MAC for ERCP point to numerous retrospective and prospective studies—mainly from the gastroenterology literature—which conclude that the technique is safe and effective.4,6,8,33,34 In a prospective study comparing MAC to GEA, Berzin et al6 reported an overall rate of SRAEs of 21%. Specific adverse events in the MAC cohort included hypoxemia (12.5%; defined as oxygen saturation <85%), unplanned mask ventilation (0.6%), unplanned intubation (3%), and procedure interruption (5%).6 From these data, the authors concluded that “minor sedation related events were common (21%) but lead to transient interruption of the procedure in only 5% of cases.” They casually dismissed the 3% incidence of unplanned intubation by stating that “airway access was easily obtained on the rare occasion unplanned intubation was deemed necessary.” In a similar prospective study of ERCP under MAC, Zhang et al7 found that sedation-related complications occurred in 18% of patients, with hypoxemia (defined as oxygen saturation <90% for at least 2 minutes) occurring in 9% of patients, and >33% of patients experiencing multiple hypoxemic episodes. The authors noted that the incidence of hypoxemia in their study was comparable to the hypoxemia rate in other similar studies and, thus, concluded that “sedation by anesthesia personnel for ERCP is safe.” In a retrospective review of MAC for ERCP, Yang et al9 reported an incidence of hypoxemia (defined as oxygen saturation <90%) requiring airway manipulation in 28% of cases, with 1.6% of patients requiring conversion to GEA due to food in the stomach. Despite their findings, the authors concluded that “propofol can be used safely and effectively as a sedative agent for patients undergoing ERCP.”
How can studies that report such high rates of SRAEs, hypoxemic episodes, and necessary airway maneuvers conclude that the sedation is “safe” or “feasible” or “appropriate?”4,6–9 Just because a critical event does not lead to a critical outcome, does not mean the event is any less critical! The interpretation of data ultimately relies on the lens through which they are viewed. A gastroenterologist may not be alarmed by an unplanned intubation rate of up to 3%,6 or hypoxemia rates as high as 33%,7 as long as the patient did not suffer any long-term sequelae. However, an anesthesia professional who is responsible for emergency airway management and cardiopulmonary resuscitation may view each of these hypoxemic episodes as a “near-miss” event. Keep in mind, pulse oximetry is a measure of oxygenation, not ventilation, and it cannot reliably be used to detect hypoventilation and progressive hypercarbia.35,36 Hypoxemia in the setting of supplemental oxygen use—as is standard during MAC for ERCP—is a late marker of hypoventilation and is a harbinger of impending respiratory arrest.
For the sake of argument, let’s consider a different scenario. If we drive without wearing seatbelts for a year and are never harmed in any accidents that occur, are we correct to conclude that driving without seatbelts is safe, feasible, and appropriate? Normalizing and accepting high rates of hypoxemia during MAC for ERCP, while in a remote location, in the prone position, and with limited airway access, sets a dangerous precedent. We admit that it is difficult to define an “acceptable” rate of SRAEs and hypoxemic episodes during sedation. However, in our opinion, the rates of SRAEs and hypoxemic episodes reported in the aforementioned studies are worrisome and should be presented as a patient safety concern, rather than being dismissed as an inconsequential event.
Now, let’s turn our attention toward the evidence in support of GEA for ERCP. In a randomized controlled trial comparing the safety of MAC to GEA for ERCP, the results clearly favor GEA.10 This study included patients identified to be high risk for SRAEs including those with a STOP-BANG (Scoring system involving: Snoring, Tiredness, Observed apnea, Blood Pressure, Body mass index, Age, Neck circumference, Gender) score ≥3, abdominal ascites, BMI ≥35, chronic lung disease, ASA physical status score >3, Mallampati class 4 airway, and moderate to heavy alcohol use. The rates of SRAEs were markedly higher in the MAC group compared to the GEA group (51.5% vs 9.9%).10 In the MAC group, hypoxemia (defined as oxygen saturation <90%) occurred in 19% of patients, with 45% requiring one or more airway maneuvers and 8% requiring bag-mask ventilation.10 Conversely, there were zero incidents of hypoxemia or airway maneuvers in the GEA group. The ERCP procedure had to be interrupted in 10.1% of the MAC group, requiring conversion to GEA for respiratory instability (8%) and retained gastric contents (2%).10 Of note, hypotension requiring a vasopressor occurred at similar rates in both groups, and there were no differences in procedure time, technical success, and patient recovery time.10
Putting the data aside for a moment, let’s step back and discuss the reality of crisis management from an anesthesia professional’s perspective. Airway compromise in the prone position, while isolated in a remote location, and with limited help and resources is every anesthesia professional’s nightmare—as it should be. When every second matters, it may feel like an eternity to withdraw the endoscope, move the fluoroscopy equipment out of the way, bring the stretcher into the room, and turn the patient supine. By the time the patient is appropriately positioned to manage the airway, they may be on the verge of respiratory arrest. Yes, this is a relatively rare event during sedation for ERCP, but it is preventable. Why take this risk when the airway could be secured initially with endotracheal intubation in an elective, controlled manner? With the high rates of hypoxemia associated with sedation during ERCP and the numerous challenges associated with unplanned intubation in this environment, GEA is simply the logical choice.
There is a perception among gastroenterologists that MAC is quicker than GEA, requires less turnover time, and enables higher patient throughput. Although some data exist to support this perception,5 other data suggest that any time saved during sedation is likely offset by frequent procedural interruptions due to airway compromise.10 In reality, GI suite efficiency is a complex product of many different variables (including procedural efficiency by the endoscopist), and it is shortsighted to think that efficiency is solely related to the presence or absence of an endotracheal tube. There is also a perception that MAC is inherently gentler, safer, and less invasive than GEA. Yes, the use of GEA introduces its own risks, including the potential for dental injury, residual neuromuscular blockade, hemodynamic instability, and adverse drug reactions. However, when comparing all of these risks with the risk of airway compromise during MAC for ERCP in the prone position, there frankly is no comparison. Our job as anesthesia professionals is to mitigate risk, and the potential for airway compromise during MAC for ERCP is a risk not worth taking.
Until further large scale, multi-center randomized controlled trials are conducted, the controversy regarding MAC versus GEA for ERCP will persist, and the standard of care will remain undefined. What all anesthesia professionals can agree on, however, is that regardless of the anesthetic technique, the anesthesia should be administered by a qualified anesthesia professional. In the United States, EDS for ERCP decreased from >50% of cases in 2005 to 5% in 2014, but it remains prevalent in Europe and other countries.3 A retrospective review of nearly 27,000 ERCPs performed over a 10-year span showed that EDS resulted in a higher rate of adverse events (OR = 1.86) and was nearly twice as likely to require an unplanned intervention than anesthesia-provided sedation.3 Studies also demonstrated that EDS led to a higher rate of sedation failure, and consequently procedural failure, than anesthesia-administered MAC or GEA.30,34 To make matters worse, EDS resulted in both poor patient satisfaction and poor endoscopist satisfaction.33 In our opinion, the EDS model for ERCP is a threat to patient safety and should be abandoned. We strongly believe that propofol sedation should only be administered by a qualified anesthesia professional equipped with the ability to quickly recognize airway compromise and the skills to manage an airway in the event of emergency. These skills fall outside the scope of practice of gastroenterology physicians, nurses, and technicians.
MAC anesthesia during ERCP is associated with high rates of hypoxemia, airway maneuvers, and SRAEs. These risks coupled with the inherent dangers of anesthesia in remote locations raise significant concern about the safety of MAC for ERCP in the prone position. To quote the wise anesthesiologist Dr. Carl Hug Jr, perhaps MAC should stand for “Maximal Anesthesia Caution” rather than “Monitored Anesthesia Care.”37 We believe that all patients undergoing ERCP procedures should be under the care of a qualified anesthesia professional and that GEA offers significant advantages over MAC.
SUMMARY
This Pro-Con article was prompted by the growth in complex endoscopy procedures over recent years coupled with the lack of large randomized controlled trials to support a definitive anesthetic technique for patients having ERCP. The debate is particularly important because of the incidence of comorbidities and because the procedure involves a shared airway. The benefits of MAC include fewer hemodynamic perturbations, decreased side effects from inhalation agents, faster cognitive recover, and shorter overall procedural time, which must be weighed against the incidence of critical events due to impaired oxygenation and/or ventilation known to occur during MAC. The 2 approaches highlighted in this discussion emphasize the importance of having a qualified anesthesia professional determine the optimal anesthetic for a particular patient and clinical circumstance.
References
- Woodward ZG, Urman RD, Domino KB. Safety of non-operating room anesthesia: a closed claims update. Anesthesiol Clin. 2017;35:569–581.
- Smith ZL, Das KK, Kushnir VM. Anesthesia-administered sedation for endoscopic retrograde cholangiopancreatography: monitored anesthesia care or general endotracheal anesthesia? Curr Opin Anaesthesiol. 2019;32:531–537.
- Smith ZL, Nickel KB, Olsen MA, et al. Type of sedation and the need for unplanned interventions during ERCP: analysis of the clinical outcomes research initiative national endoscopic database (CORI-NED). Frontline Gastroenterol. 2020;11:104–110.
- Barnett SR, Berzin T, Sanaka S, et al. Deep sedation without intubation for ERCP is appropriate in healthier, non-obese patients. Dig Dis Sci. 2013;58:3287–3292.
- Perbtani YB, Summerlee RJ, Yang D, et al. Impact of endotracheal intubation on interventional endoscopy unit efficiency metrics at a tertiary academic medical center. Am J Gastroenterol. 2016;111:800–807.
- Berzin TM, Sanaka S, Barnett SR, et al. A prospective assessment of sedation-related adverse events and patient and endoscopist satisfaction in ERCP with anesthesiologist-administered sedation. Gastrointest Endosc. 2011;73:710–717.
- Zhang CC, Ganion N, Knebel P, et al. Sedation-related complications during anesthesiologist-administered sedation for endoscopic retrograde cholangiopancreatography: a prospective study. BMC Anesthesiol. 2020;20:131.
- Coté GA, Hovis RM, Ansstas MA, et al. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010;8:137–142.
- Yang JF, Farooq P, Zwilling K, et al. Efficacy and safety of propofol-mediated sedation for outpatient endoscopic retrograde cholangiopancreatography (ERCP). Dig Dis Sci. 2016;61:1686–1691.
- Smith ZL, Mullady DK, Lang GD, et al. A randomized controlled trial evaluating general endotracheal anesthesia versus monitored anesthesia care and the incidence of sedation-related adverse events during ERCP in high-risk patients. Gastrointest Endosc. 2019;89:855–862.
- Metzner J, Posner KL, Domino KB. The risk and safety of anesthesia at remote locations: the US closed claims analysis. Curr Opin Anaesthesiol. 2009;22:502–508.
- Wu WZ, Zheng MH, Wang JC, Chen S. The role of endoscopic retrograde cholangiopancreatography in perioperative period of laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2002;1:114–117.
- Kapoor H. Anaesthesia for endoscopic retrograde cholangiopancreatography. Acta Anaesthesiol Scand. 2011;55:918–926.
- Huang RJ, Barakat MT, Girotra M, et al. Unplanned hospital encounters after endoscopic retrograde cholangiopancreatography in 3 large North American States. Gastroenterology. 2019;156:119.e3–129.e3.
- Goudra B, Singh PM. Airway management during upper GI endoscopic procedures: state of the art review. Dig Dis Sci. 2017;62:45–53.
- Shah T, Ianchulev S. Gastroscope-facilitated endotracheal intubation during ERCP: when is the best time to GETA (Big) MAC? Dig Dis Sci. 2021;66:938–940.
- Barakat MT, Angelotti TP, Banerjee S. Use of an ultra-slim gastroscope to accomplish endoscopist-facilitated rescue intubation during ERCP: a novel approach to enhance patient and staff safety. Dig Dis Sci. 2021;66:1285–1290.
- Standards for Basic Anesthetic Monitoring. American Society of Anesthesiologist Website. October 21, 1986, last amended on October 20, 2010, and last affirmed on October 28, 2016. https://www.asahq.org/~/media/Sites/ASAHQ/Files/Public/Resources/standards-guidelines/standards-for-basic-anesthetic-monitoring.pdf. Accessed March 20, 2021.
- Deitch K, Miner J, Chudnofsky CR, et al. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55:258–264.
- Goudra B, Singh PM. ERCP: the unresolved question of endotracheal intubation. Dig Dis Sci. 2014;59:513–519.
- Gambee AM, Hertzka RE, Fisher DM. Preoxygenation techniques: comparison of three minutes and four breaths. Anesth Analg. 1987;66:468–470.
- Tetzlaff JE, Vargo JJ, Maurer W. Nonoperating room anesthesia for the gastrointestinal endoscopy suite. Anesthesiol Clin. 2014;32:387–394.
- Goulson DT, Fragneto RY. Anesthesia for gastrointestinal endoscopic procedures. Anesthesiol Clin. 2009;27:71–85.
- Allampati S, Wen S, Liu F, Kupec JT. Recovery of cognitive function after sedation with propofol for outpatient gastrointestinal endoscopy. Saudi J Gastroenterol. 2019;25:188–193.
- Berg H, Roed J, Viby-Mogensen J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–1103.
- Althoff FC, Agnihotri A, Grabitz SD, et al. Outcomes after endoscopic retrograde cholangiopancreatography with general anaesthesia versus sedation. Br J Anaesth. 2021;126:191–200.
- Coelho-Prabhu N, Shah ND, Van Houten H, et al. Endoscopic retrograde cholangiopancreatography: utilisation and outcomes in a 10-year population-based cohort. BMJ Open. 2013;3:e002689.
- Cotton PB, Garrow DA, Gallagher J, Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80–88.
- Chandrasekhara V, Khashab MA, Muthusamy R, et al. Committee ASoP. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32–47.
- Raymondos K, Panning B, Bachem I, et al. Evaluation of endoscopic retrograde cholangiopancreatography under conscious sedation and general anesthesia. Endoscopy. 2002;34:721–726.
- Wehrmann T, Kokabpick S, Lembcke B, et al. Efficacy and safety of intravenous propofol sedation during routine ERCP: a prospective, controlled study. Gastrointest Endosc. 1999;49:677–683.
- Lapidus A, Gralnek IM, Suissa A, et al. Safety and efficacy of endoscopist-directed balanced propofol sedation during endoscopic retrograde cholangiopancreatography. Ann Gastroenterol. 2019;32:303–311.
- Goudra BG, Singh PM, Gouda G, et al. Safety of nonanesthesia provider-administered propofol (NAAP) sedation in advanced gastrointestinal endoscopic procedures: comparative meta-analysis of pooled results. Dig Dis Sci. 2015;60:2612–2627.
- Buxbaum J, Roth N, Motamedi N, et al. Anesthetist-directed sedation favors success of advanced endoscopic procedures. Am J Gastroenterol. 2017;112:290–296.
- Fu ES, Downs JB, Schweiger JW, et al. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126:1552–1558.
- Hutton P, Clutton-Brock T. The benefits and pitfalls of pulse oximetry. BMJ. 1993;307:457–458.
- Hug CC Jr. MAC should stand for maximum anesthesia caution, not minimal anesthesiology care. Anesthesiology. 2006;104:221–223.