The American Society of Anesthesiologists (ASA) Committee on Practice Parameters (CPP), chaired by Karen Domino, MD, MPH, created a task force to develop guidelines for neuromuscular blockade (NMB) to improve patient safety and satisfaction. The Anesthesia Patient Safety Foundation (APSF) and its leadership have long advocated for guidelines on the use of NMB, its monitoring, and reversal, given the patient safety risk of residual muscle weakness. The task force, co-chaired by Stephan Thilen, MD, MS, and Wade Weigel, MD, developed the 2023 ASA Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade, which were published in a January issue of Anesthesiology.1 This article will provide an overview of the new guidelines.a
aDisclaimer: ASA practice guidelines aim to improve patient care, safety, and outcomes by providing up-to-date information for patient care. Practice guidelines are subject to revision as warranted by the evolution of medical knowledge, technology, and practice. Practice guidelines are not intended as standards or absolute requirements to replace local institutional policies, and their use cannot guarantee any specific outcome.1
The practice guidelines present eight recommendations regarding the type of monitoring of neuromuscular blockade, location of monitoring, and medications used to achieve appropriate reversal of neuromuscular blockade. Six recommendations (1–6) were classified as strong recommendations with moderate strength of evidence. The two remaining recommendations (7–8) were classified as conditional recommendations with low and very low strength of evidence, respectively.
Neuromuscular blocking drugs are commonly used and have been shown in the literature to be associated with an incidence of residual blockade at the end of surgery and/or in the postanesthesia care unit (PACU) of up to 64%.2,3 Residual blockade is associated with numerous complications, such as upper airway obstruction, reintubation, atelectasis, pneumonia, prolonged PACU stay, and decreased patient satisfaction.4-7
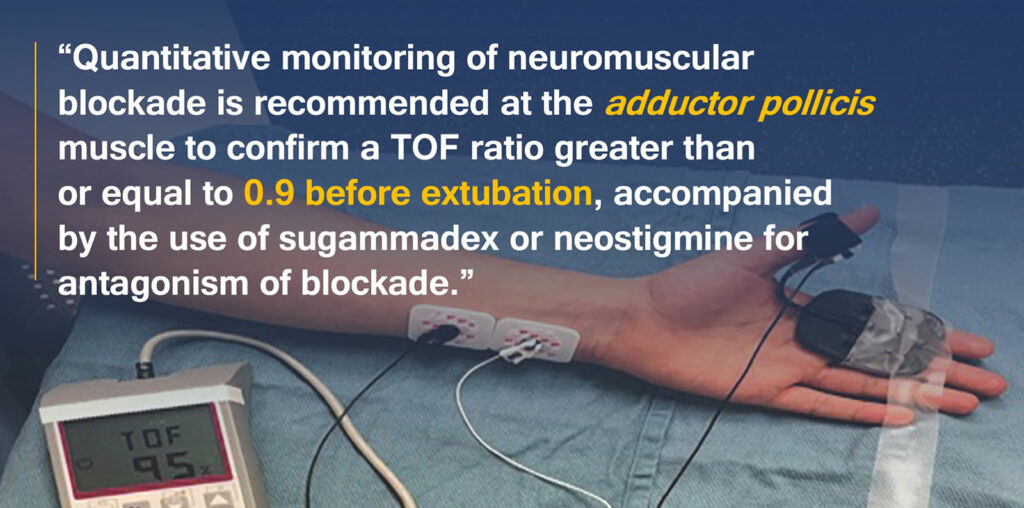
Quantitative assessment of neuromuscular blockade can be performed with peripheral nerve stimulators that deliver four brief electrical pulses. The amplitude of the fourth twitch divided by the amplitude of the first twitch results in a train-of-four (TOF) ratio. The baseline TOF ratio in the unparalyzed patient should be 1.0, indicating all four twitches have equal amplitude. The smaller the TOF ratio, the greater the degree of paralysis. There is a broad consensus that acceptable recovery of neuromuscular function is defined as a TOF ratio greater than or equal to 0.9.1 However, despite multiple studies reporting significant benefits of quantitative monitoring of neuromuscular blockade, it has not been widely adopted among all anesthesia professionals.1 A 2019 international survey identifies several factors that have contributed to the slow adoption of quantitative monitoring: anesthesia professionals’ overconfidence in the assessment of neuromuscular blockade depth, an underappreciation of the frequency of residual neuromuscular blockade and its clinical consequences, and a lack of commercially available quantitative TOF monitors that are user-friendly and inexpensive.8
Qualitative assessment of neuromuscular blockade is more frequently used by anesthesia professionals.1 Following peripheral nerve stimulation, one performs visual inspection or manual (tactile) evaluation for subjective assessment of thumb movement, resulting in a TOF count. However, studies have shown that clinically significant weakness cannot be identified with this technique, as fade cannot be reliably appreciated until the TOF ratio is less than approximately 0.4.9 Another common approach is subjective assessment of sustained head lift or grip strength. However, studies have also shown that these maneuvers are not sensitive enough to detect residual neuromuscular blockade, as 80% of patients with a TOF ratio < 0.7 could perform a head lift maneuver.10
Moreover, the duration of action of neuromuscular blocking drugs has great interpatient variability, and it is not possible to use time intervals to predict when the block has regressed to a specific depth of block. The practice guidelines cite 11 studies that were pooled and analyzed, reporting lower incidences of residual neuromuscular blockade with quantitative monitoring compared with qualitative or clinical assessment (Supplemental Tables S8 and S9 (Note: link downloads a Word doc), https://links.lww.com/ALN/C928).1 Therefore, when neuromuscular blocking drugs are administered, clinical assessment alone is not recommended to avoid residual neuromuscular blockade (Recommendation 1), and quantitative monitoring is recommended over qualitative assessment to reduce the risk of residual neuromuscular blockade (Recommendation 2).1
Residual neuromuscular blockade was initially defined as a TOF ratio less than 0.7, based on earlier work showing that vital capacity and inspiratory force had recovered to near normal at this ratio,11 but numerous later studies have shown that patients have clinical symptoms of weakness with a train of four ratio less than 0.9.12 As mentioned, the practice guidelines recommend using quantitative TOF monitoring, and the guidelines specifically recommend confirming a TOF ratio greater than or equal to 0.9 before extubation, as there is a lower incidence of residual neuromuscular blockade compared to when the TOF ratio was not confirmed to recover to this level (Recommendation 3).1
Of note, various types of quantitative TOF monitors exist, such as acceleromyography, electromyography, kinemyography, and mechanomyography. The guidelines present two supplemental tables that summarize the last 30 years of data regarding the agreement among technologies (bias) as TOF differences at a given TOF ratio (Supplemental Table 24 (Note: link downloads a Word doc), https://links.lww.com/ALN/C928) and as time to attain a given TOF ratio (Supplemental Table 26 (Note: link downloads a Word doc), https://links.lww.com/ALN/C928). These data indicate there are differences among technologies (a discussion of which is beyond the scope of this article), but the guidelines state there is no preferred type of quantitative neuromuscular monitor.1
The practice guidelines state that acceptable recovery of all muscles from neuromuscular blockade optimizes patient safety, and therefore, measurements “should be obtained at sites with longer times to recovery.”1 Studies have shown that eye muscles (corrugator supercilii and orbicularis oculi) are relatively resistant to neuromuscular blocking drugs compared to the adductor pollicis muscle.1 Therefore, the time to reach a TOF ratio greater than or equal to 0.9 at the adductor pollicis muscle was longer than the time to reach this threshold at the eye muscles (Supplemental Tables S15 and S16 (Note: link downloads a Word doc), https://links.lww.com/ALN/C928).1 Therefore, it is recommended to use the adductor pollicis muscle for neuromuscular monitoring (Recommendation 4), and it is recommended to avoid using eye muscles for neuromuscular monitoring (Recommendation 5).1 The guidelines also state that if intraoperative neuromuscular monitoring has been performed at the eye muscles because no other site was easily accessible intraoperatively, then changing the site to the adductor pollicis muscle before antagonism is recommended.1
Efficacious pharmacologic antagonism of neuromuscular blockade depends on the depth of blockade. The practice guidelines use the same scheme for classification of different depths of block presented in the 2018 Consensus Statement on Perioperative Use of Neuromuscular Monitoring (Table 1).1,13 Aminosteroid induced neuromuscular blockade can be antagonized in two ways. Anticholinesterases inhibit acetylcholinesterase and butyrylcholinesterase, prolonging the presence of acetylcholine at the neuromuscular junction. Neostigmine was the only anticholinesterase that was evaluated in the practice guidelines, as edrophonium is no longer available in the United States. Sugammadex is a selective relaxant binding agent, and it can antagonize any depth of block that is induced by rocuronium or vecuronium. It is more efficacious than neostigmine for antagonism of deep, moderate, and shallow levels of block and is recommended for antagonism of these depths of neuromuscular blockade (Recommendation 6).1 The FDA-approved dose recommendations for antagonizing rocuronium or vecuronium with sugammadex are 2 mg/kg for TOF count = 2 to TOF ratio < 0.9, 4 mg/kg for posttetanic count = 1 to TOF count = 1, and 16 mg/kg for immediate antagonism after administration of a single dose of rocuronium 1.2mg/kg.14
Table 1: Depths of Neuromuscular Blockade by Quantitative and Qualitative Measurement.
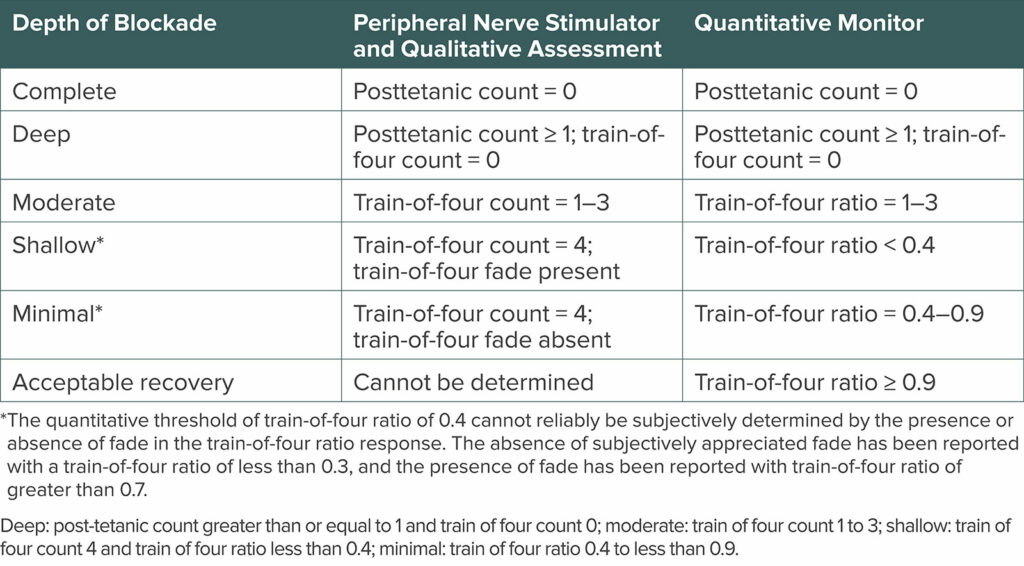
Table 5 from the 2023 ASA Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade: A Report by the American Society of Anesthesiologists Task Force on Neuromuscular Blockade.1 Reprinted and modified with permission from Wolters Kluwer Health, Inc.
Neostigmine is efficacious for antagonism of minimal block (TOF ratio ≥ 0.4 to < 0.9), and it is recommended as a reasonable alternative to sugammadex for antagonism of minimal block (Recommendation 7).1 If neostigmine is used for antagonism of a block that is deeper than minimal blockade, the degree of antagonism will vary between patients. If qualitative assessment is used, it is not possible to determine when recovery to a TOF ratio ≥ 0.9 is attained. The guidelines include a comment on this situation: “Depending on clinical judgment and in the context of quantitative monitoring, neostigmine may be considered for a depth of block deeper than minimal (TOF ratio of 0.4 to 0.9), with the understanding that deeper blocks will require more time to attain a TOF ratio greater than or equal to 0.9.”1
Studies examining the adverse effects of sugammadex and neostigmine (co-administered with glycopyrrolate) do not favor either drug. The practice guidelines cite more than 75 studies that did not detect a difference between sugammadex and neostigmine in the incidence of pulmonary complications, anaphylaxis, bradycardia, or tachycardia (when administered with glycopyrrolate), postoperative nausea alone, and postoperative vomiting.
Benzylisoquinolinium neuromuscular blocking drugs, such as atracurium and cisatracurium, can only be antagonized by acetylcholinesterase inhibitors. The antagonist effect of neostigmine, the most used acetylcholinesterase inhibitor, is maximal within 10 minutes.15 Moreover, neostigmine’s efficacy is significantly improved when antagonizing minimal block compared to deeper levels of block. Therefore, Recommendation 8 states that to avoid residual neuromuscular blockade when qualitative assessment is used, antagonism of a cisatracurium- or atracurium-induced block should not be initiated before there is absence of subjectively assessed fade in the train of four response and at least 10 minutes should elapse from antagonism with neostigmine to extubation.1 When quantitative monitoring is used, extubation can be done as soon as a train of four ratio greater than or equal to 0.9 is confirmed.
SUMMARY OF RECOMMENDATIONS1
- When neuromuscular blocking drugs are administered, we recommend against clinical assessment alone to avoid residual neuromuscular blockade, due to the insensitivity of the assessment.1
- We recommend quantitative monitoring over qualitative assessment to avoid residual neuromuscular blockade.
- When using quantitative monitoring, we recommend confirming a train of four ratio greater than or equal to 0.9 before extubation.
- We recommend using the adductor pollicis muscle for neuromuscular monitoring.
- We recommend against using eye muscles for neuromuscular monitoring.
- We recommend sugammadex over neostigmine at deep, moderate, and shallow depths of neuromuscular blockade induced by rocuronium or vecuronium, to avoid residual neuromuscular blockade.
- We suggest neostigmine as a reasonable alternative to sugammadex at minimal depth of neuromuscular blockade.
- To avoid residual neuromuscular blockade when atracurium or cisatracurium are administered and qualitative assessment is used, we suggest antagonism with neostigmine at minimal neuromuscular blockade depth. In the absence of quantitative monitoring, at least 10 minutes should elapse from antagonism to extubation. When quantitative monitoring is utilized, extubation can be done as soon as a train of four ratio greater than or equal to 0.9 is confirmed before extubation.
CONCLUSION
Residual neuromuscular blockade is an important patient safety issue, and recently published practice guidelines present eight recommendations for the monitoring and antagonism of neuromuscular blockade in the United States that are supported in the literature. Quantitative monitoring of neuromuscular blockade is recommended at the adductor pollicis muscle to confirm a TOF ratio greater than or equal to 0.9 before extubation, accompanied by the use of sugammadex or neostigmine for antagonism of blockade. Recognizing that quantitative monitoring may not be available in all practice settings, qualitative monitoring of the TOF count can guide dosages and timing of reversal agents of neuromuscular blocking drugs.
REFERENCES
- Thilen SR, Weigel WA, Todd MM, et al. 2023 American Society of Anesthesiologists practice guidelines for monitoring and antagonism of neuromuscular blockade: a report by the American Society of Anesthesiologists Task Force on Neuromuscular Blockade. Anesthesiology. 2023; 138:13–41. PMID: 36520073
- Fortier LP, McKeen D, Turner K, et al. The RECITE Study: A Canadian prospective, multicenter study of the incidence and severity of residual neuromuscular blockade. Anesth Analg. 2015;121:366–372. PMID: 25902322
- Saager L, Maiese EM, Bash LD, et al. Incidence, risk factors, and consequences of residual neuromuscular block in the United States: the prospective, observational, multicenter RECITE-US study. J Clin Anesth. 2019;55:33–41. PMID: 30594097
- Berg H, Roed J, Viby-Mogensen J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–1103. PMID: 9366929
- Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology. 2010;112:1013–1022. PMID: 20234315
- Butterly A, Bittner EA, George E, et al. Postoperative residual curarization from intermediate-acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth. 2010;105:304–309. PMID: 20576632
- Murphy GS, Szokol JW, Avram MJ, et al. Intraoperative acceleromyography monitoring reduces symptoms of muscle weakness and improves quality of recovery in the early postoperative period. Anesthesiology. 2011;115:946–54. PMID: 21946094
- Naguib M, Brull SJ, Hunter JM, et al. Anesthesiologists’ overconfidence in their perceived knowledge of neuromuscular monitoring and its relevance to all aspects of medical practice: an international survey. Anesth Analg. 2019;128:1118–1126. PMID: 31094776
- Viby-Mogensen J, Jensen NH, Engbaek J, et al. Tactile and visual evaluation of the response to train-of-four nerve stimulation. Anesthesiology. 1985;63:440–443. PMID: 4037404
- Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology. 2003;98:1042–1048. PMID: 12717123
- Ali HH, Wilson RS, Savarese JJ, Kitz RJ. The effect of tubocurarine on indirectly elicited train-of-four muscle response and respiratory measurements in humans. Br J Anaesth. 1975;47:570–574. PMID: 1138775
- Kopman AF, Yee PS, Neuman GG. Relationship of the train-of-four fade ratio to clinical signs and symptoms of residual paralysis in awake volunteers. Anesthesiology. 1997; 86:765–771. PMID: 9105219
- Naguib M, Brull SJ, Kopman AF, et al. Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg. 2018;127:71–80. PMID: 29200077
- FDA Prescribing information for sugammadex. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022225lbl.pdf Accessed on April 10, 2023.
- Miller R, Van Nyhuis L, Eger EI, et al. Comparative times to peak effect and durations of action of neostigmine and pyridostigmine. Anesthesiology. 1974;41:27–33. PMID: 4834375