 When patients reach the postanesthesia care unit after undergoing major noncardiac surgery, families assume they have naturally survived the most dangerous part of the perioperative experience. Their assumption is wrong. Mortality in the 30 days after surgery is more than 100 times higher than intraoperative mortality.1,2 In fact, if the month after surgery were considered a disease, it would be the third leading cause of death in the United States.3
When patients reach the postanesthesia care unit after undergoing major noncardiac surgery, families assume they have naturally survived the most dangerous part of the perioperative experience. Their assumption is wrong. Mortality in the 30 days after surgery is more than 100 times higher than intraoperative mortality.1,2 In fact, if the month after surgery were considered a disease, it would be the third leading cause of death in the United States.3
Three-quarters of postoperative mortality occurs during the initial hospitalization, that is, under direct medical care in our highest-level facilities.4 The two most common and comparable causes of 30-day mortality after noncardiac surgery are major bleeding and myocardial injury.5,6
Myocardial Injury
Myocardial infarction (MI), per 4th Universal Definition, is defined by troponin elevation and either symptoms or signs of myocardial ischemia.7 Myocardial injury after non-cardiac surgery (MINS) is defined by troponin elevation of presumably ischemic origin, and is highly associated with 30-day8 and one-year9 mortality. MINS includes myocardial infarction and other ischemic myocardial injuries not fulfilling the definition of myocardial infarction.
Perioperative myocardial injury is generally a Type-2 event, resulting largely from supply-demand mismatch. MINS and perioperative myocardial infactions thus differ from nonoperative infarctions, which usually result from plaque rupture. Soberingly, mortality from perioperative myocardial events is higher than for nonoperative infarctions—and thus deserves considerable attention.10,11
Troponin Screening
More than 90% of MINS and MI occurs within the initial two postoperative days, and more than 90% are asymptomatic.12 While it is tempting to dismiss asymptomatic troponin elevations as “troponitis,” mortality is nearly as high without symptoms as with symptoms (Figure 1). MINS should thus be taken as seriously as classical symptomatic infarctions.
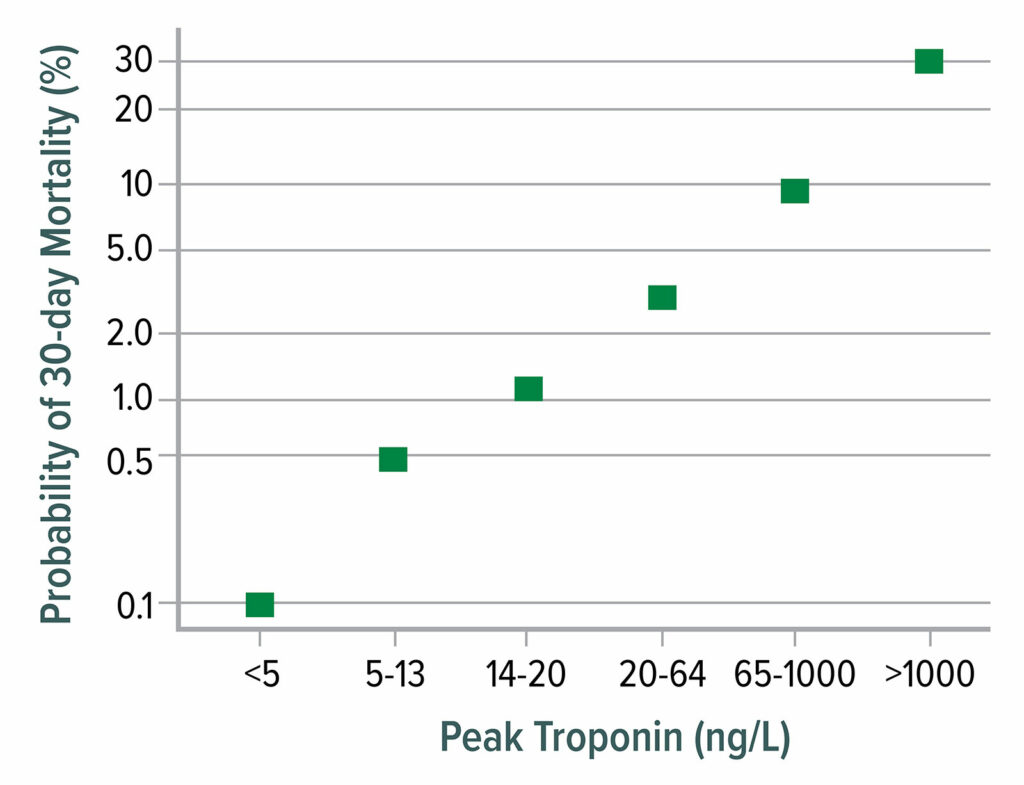
Figure 1: 30-day mortality as a function of postoperative peak high-sensitivity troponin T. Mortality increases markedly from 0.1% at a troponin T concentration <5 ng/L to 30% mortality when troponin T exceeds 1,000 ng/L.
Data from Writing Committee for the Vision Study Investigators: Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery.12
This figure is adapted from data presented in reference 12.
In the absence of routine troponin screening, most myocardial injury is missed. A reasonable strategy is to measure troponin preoperatively and for the first three postoperative days. The thresholds for MINS differ depending on the assay generation and type:
- non-high-sensitivity (fourth-generation) troponin T ≥0.03 ng/ml4;
- high-sensitivity troponin T ≥65 ng/L; or high-sensitivity troponin T=20–64 ng/L and an increase ≥5 ng/L from baseline12;
- high-sensitivity troponin I (Abbott assay [Abbott Park, IL]) ≥60 ng/L13;
- high-sensitivity troponin I (Siemens assay [Munich, Germany]) ≥75 ng/L (Borges, unpublished);
- troponin I (other assays) is at least twice local 99th percentiles;
- an increase of at least 20% in patients who have preoperative high-sensitivity troponin concentrations that exceed 80% of the relevant thresholds in items 2–5.
Hypotension
Both MINS and MI are strongly associated with many unmodifiable baseline characteristics including age, diabetes, and cardiovascular history. Large randomized trials (n=7,000–10,000) have shown that MI cannot be safely prevented by beta blockers,14 avoiding nitrous oxide,15 by clonidine,16 or aspirin.17 In a recent large trial, one patient in seven who had MINS had a major vascular event (mostly re-infarctions) within 17 postoperative months.11
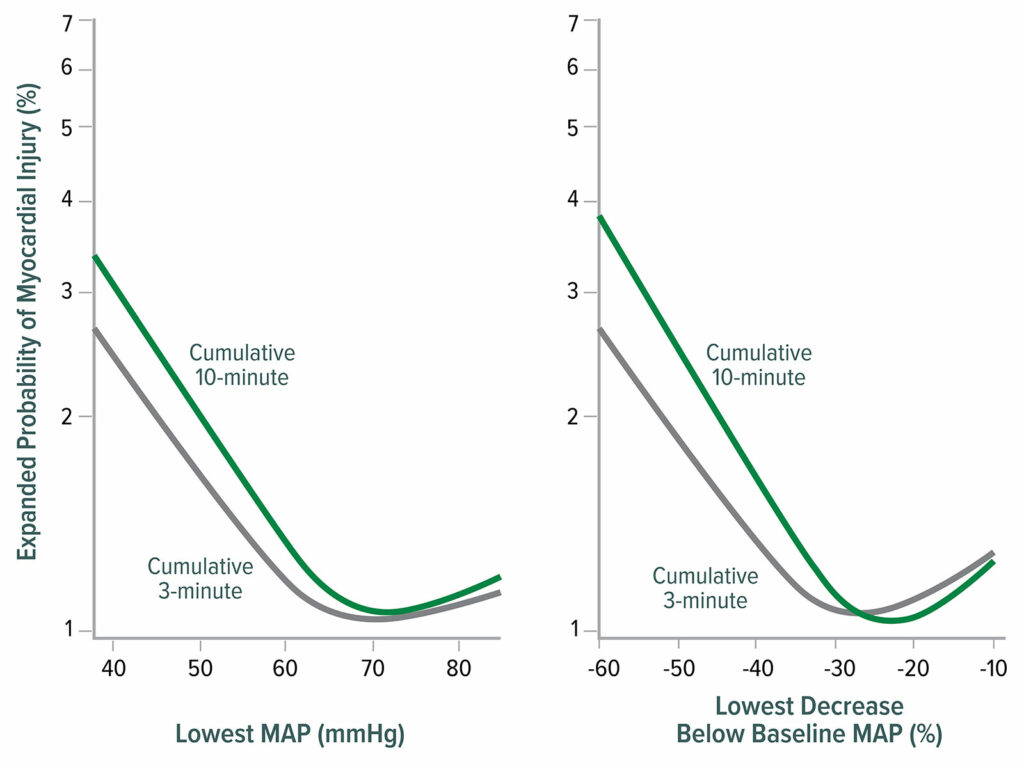
Intraoperative hypotension is associated with MINS and MI, with the harm threshold being a mean arterial pressure (MAP) ≈65 mmHg (Figure 2).18,19 Postoperative hypotension is also associated with myocardial infarction, independent of intraoperative hypotension (Figure 3).20,21
Figure 2: Lowest mean arterial pressure (MAP) thresholds for myocardial injury after noncardiac surgery. The left graph shows the relationship between the lowest cumulative absolute mean arterial pressure maintained for 3 and 10 minutes and myocardial injury. The right graph shows the relationship between the lowest cumulative relative mean arterial pressure maintained for 3 and 10 minutes and myocardial injury. Both graphs are multivariable logistic regressions adjusted for baseline characteristics.18
Reproduced and modified with permission. Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, Kurz A. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology.2017;126:47–65.
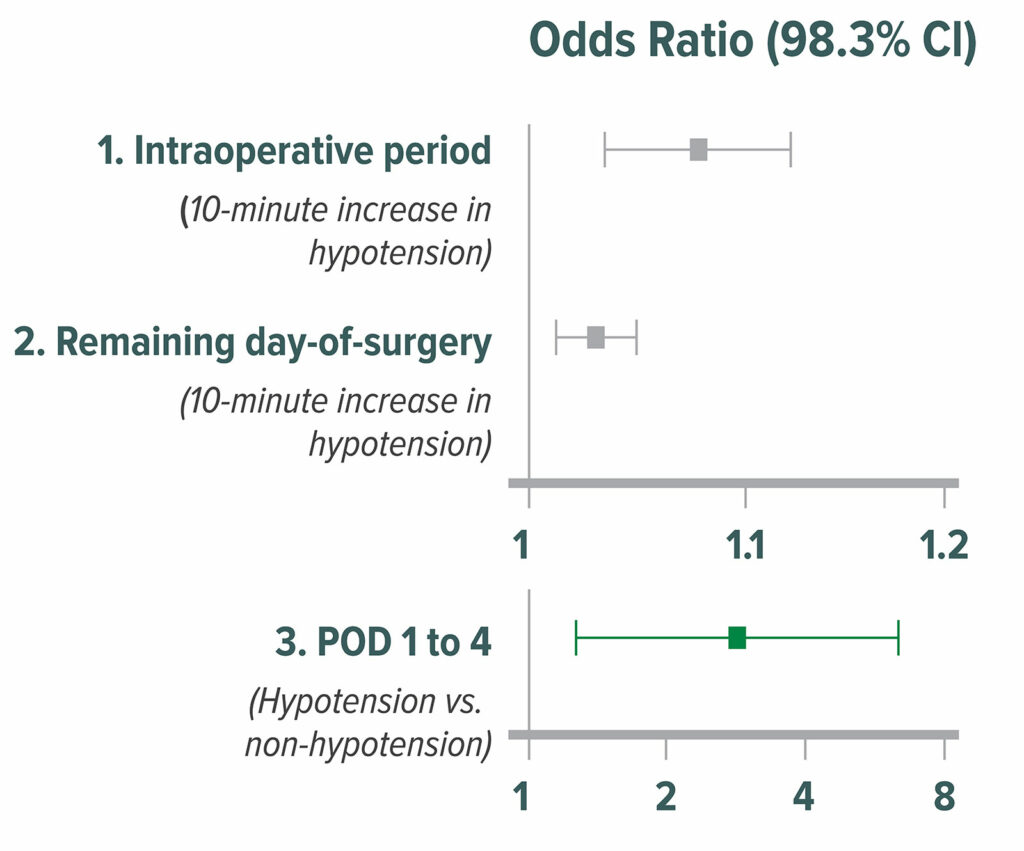
Figure 3: Odds ratios of average relative effect on the primary composite of 30-day myocardial infarction and mortality for three perioperative periods: intraoperative, remaining day of surgery, and the initial four PODs of hospitalization. CIs for multiple comparisons were adjusted by Bonferroni correction. Correspondingly, P<0.017 (0.05/3) was considered to be significant for the average relative effect. The squares present the odds ratios, and the bars present the CIs. POD = postoperative day.20
Reproduced and modified with permission. Sessler DI, Meyhoff CS, Zimmerman NM, Mao G, Leslie K, Vasquez SM, Balaji P, Alvarez-Garcia J, Cavalcanti AB, Parlow JL, Rahate PV, Seeberger MD, Gossetti B, Walker SA, Premchand RK, Dahl RM, Duceppe E, Rodseth R, Botto F, Devereaux PJ. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE-2 trial. Anesthesiology. 2018;128:317–327.
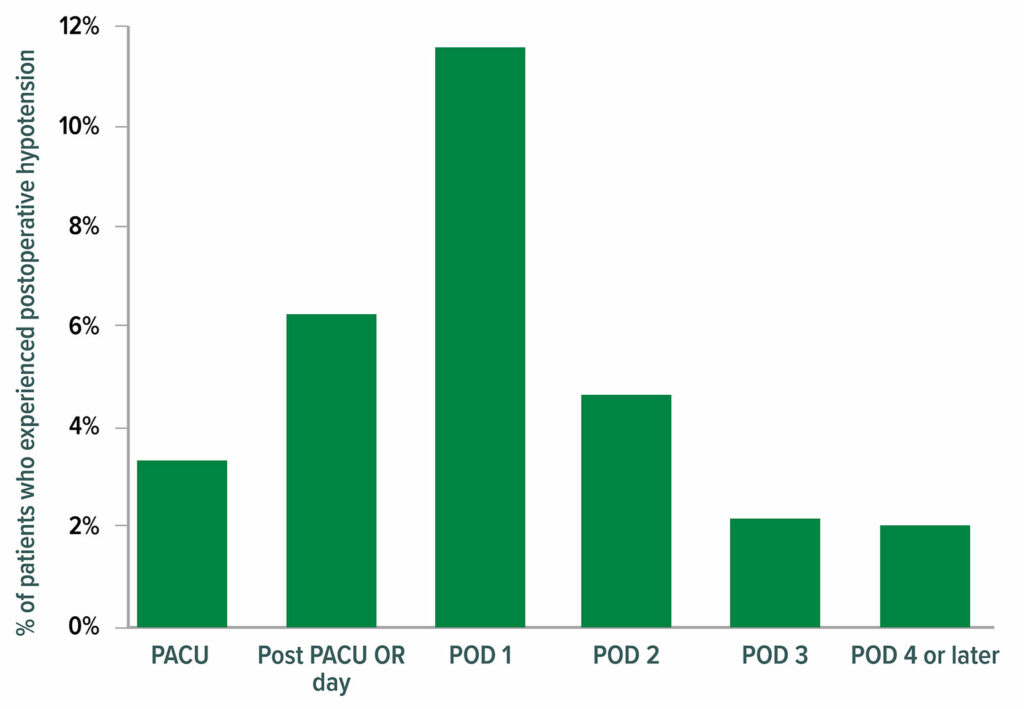
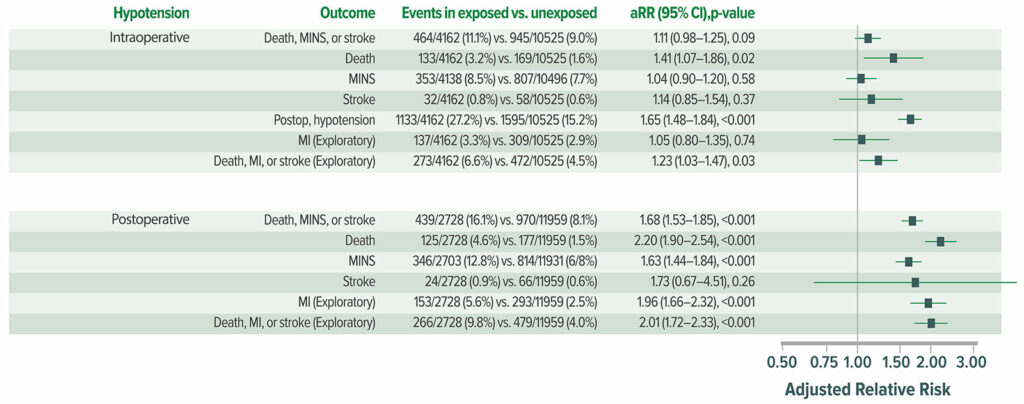
Results from the VISION cohort show that postoperative hypotension is common (Figure 4) and strongly associated with major vascular events. Postoperative hypotension is more strongly associated with myocardial infarction and/or death than intraoperative hypotension (Figure 5).22 Perioperative hypotension is also associated with stroke,14,22-25 although inconsistently.26
Figure 4: Clinically meaningful hypotension (systolic pressure <90 and prompting intervention). In total, 2,860 of 14,687 patients (19.5%) experienced at least one episode of clinically meaningful hypotension after their surgery; 2,728 (95.4%) of those patients experienced a hypotensive episode by postoperative day (POD) 3. OR = operating room; PACU = postanesthesia care unit.22
Reproduced and modified with permission. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126:16–27.
Figure 5: Adjusted association between hypotension and postoperative death and vascular events in all 14,687 patients. aRR = adjusted relative risk.22
Reproduced and modified with permission. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126:16–27.
Other Factors
Two recent studies identified remarkably strong associations between postoperative anemia and myocardial injury27 and infarction,28 even after adjusting for baseline patient characteristics and preoperative anemia. In contrast, a heart rate up to 100 beats/min and systolic hypertension up to 200 mmHg are not important risk factors for postoperative myocardial injury.29 General hospital floor hypoxemia is common, profound, and prolonged30; however, it remains unknown whether hypoxemia contributes to myocardial injury. Fortunately, simultaneous hospital floor hypotension and hypoxemia—which might especially provoke supply-demand injury—is rare.
Acute Kidney Injury
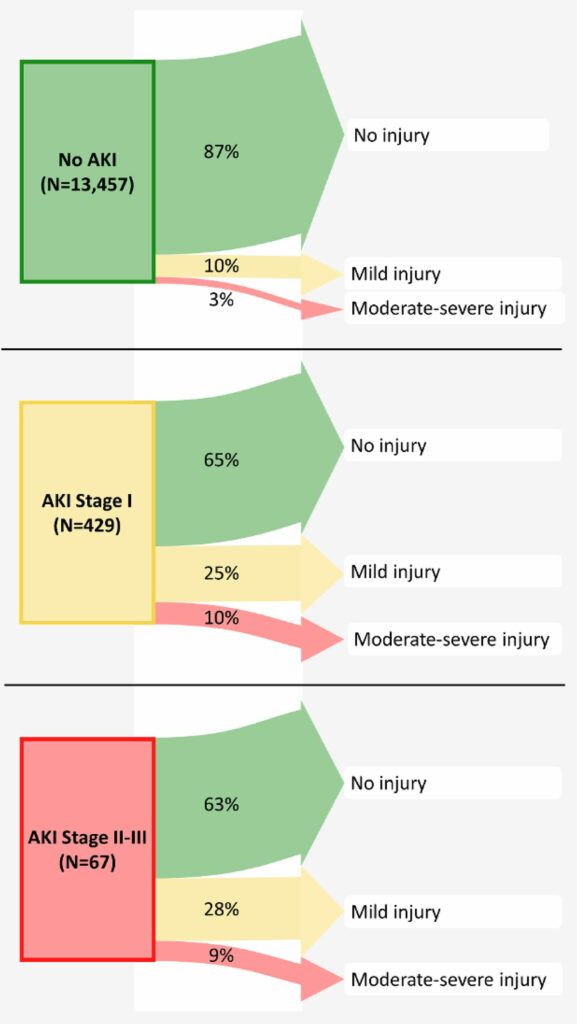
Figure 6: Renal outcomes 1–2 years after surgery, according to postoperative acute kidney injury stage. Width of the arrows represents the percentage of patients from each exposure group having each stage of long-term renal injury.37 A quarter of patients with stage I postoperative kidney injury (creatinine increase of ≥ 0.3 mg/dl or 1.5–1.9 times the baseline level) still had mild injury 1–2 years later, and 10% had even higher stage injury. A full third of the patients with stage I kidney injury thus had renal injury 1–2 years after surgery. Consequently, patients with postoperative stage I injury had an odds ratio (95%CI) of 2.3 (1.8, 2.9) for having long-term renal injury compared to patients without postoperative kidney injury. We thus conclude that in adults recovering from noncardiac surgery, even a mild postoperative increase in plasma creatinine, corresponding to stage I kidney injury, is associated with worse renal outcome 1–2 years after surgery and should therefore be considered a clinically important perioperative outcome.
Reproduced and modified with permission. Turan A, Cohen B, Adegboye J, Makarova N, Liu L, Mascha EJ, Qiu Y, Irefin S, Wakefield BJ, Ruetzler K, Sessler DI. Mild acute kidney injury after noncardiac surgery is associated with long-term renal dysfunction: a retrospective cohort study. Anesthesiology. 2020;132:1053–1061.
New-onset acute kidney injury (AKI) is common after noncardiac surgery, with stages 2–3 occurring in up to 1% of patients,31 and in up to 7.4% of patients when stage 1 AKI is included.32 There is currently no reliable way to predict AKI.33 The hypotensive harm threshold for AKI is similar or slightly higher than that for myocardial injury, presumably because the metabolic rate of the kidney is high.18,32,34
Notably, at a more stringent MAP cut-off of <55 mmHg, <5 minutes below this pressure is associated with an 18% increase in risk for AKI.34 Other analyses report similar associations.35 Taken together, these studies confirm a robust association of both degree and duration of perioperative hypotension and AKI, and hence the importance of considering both duration and excursion when quantifying hypotension.
The implications of perioperative AKI extend past the index hospitalization. In a 1,869-patient observational cohort examining the association of perioperative AKI with 1-year mortality, AKI was associated with an adjusted hazard ratio for death of 3.36 Lastly, we note that even milder degrees of AKI have lasting consequences: 37% of Stage 1 AKI persists or is worse 1–2 years after non-cardiac surgery (Figure.6).37
Delirium
Delirium is a common complication of cardiac surgery and is associated with morbidity and mortality.38-42 The reported incidence of delirium after major non-cardiac surgery is about 10%, and increases markedly as patient age increases beyond 65 years.43 The pathophysiology of delirium is multifactorial, but presumably includes inadequate brain perfusion that results when mean arterial pressure is less than the lower limit of autoregulation.44-46
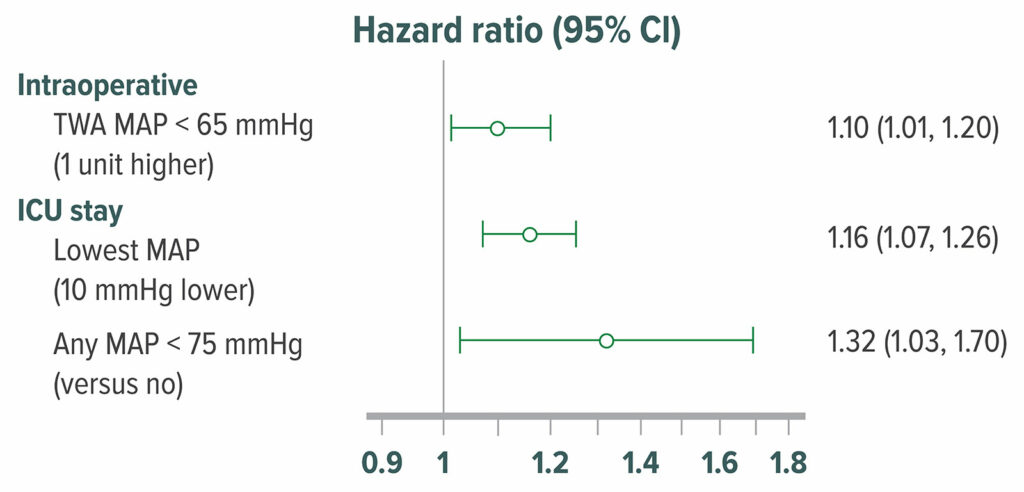
The cerebral autoregulation threshold remains unclear, but there appears to be considerable inter-individual variation, and may be as high as 85 mmHg in some patients.47,48 Consistent with this theory, hypotension is associated with delirium and cognitive decline (Figure 7),49-51 although inconsistently.52-54 Limited randomized data (n=199) indicate that hypotension causes delirium.55
Figure 7: Adjusted hazard ratio of delirium in 908 postoperative patients who were admitted directly from an operating room to the surgical intensive care unit. Delirium was assessed with the Confusion Assessment Method for Intensive Care Unit patients at 12-hour intervals. 316 (35%) patients had delirium within the first 5 postoperative days in the surgical intensive care unit. Intraoperative hypotension, MAP <65 mmHg was significantly associated with higher odds of postoperative delirium.50 TWA=Time Weighted Average
Reproduced and modififed with permission. Maheshwari K, Ahuja S, Khanna AK, Mao G, et al. Association between perioperative hypotension and delirium in postoperative critically ill patients: a retrospective cohort analysis. Anesth Analg. 2020;130:636–643.
Patients who have delirium after surgery are far more likely than others to develop long-term cognitive impairment;56 however, it remains unknown whether the association is causal. Hypotension may also provoke overt—or far more commonly—covert strokes which are strongly linked to delirium.57
Blood Pressure Management
Intraoperative hypotension cannot be reliably predicted from baseline patient characteristics or the surgical procedure.58 How best to prevent and treat perioperative hypotension remains unclear. There is remarkably little correlation between intraoperative cardiac index and blood pressure, and the assumption that maintaining adequate vascular volume prevents hypotension does not appear accurate. Furthermore, in one study, a third of all intraoperative hypotension occurred between anesthetic induction and surgical incision—and was thus obviously consequent to anesthetic drugs rather than volume shifts. Pre-incisional hypotension is as strongly associated with organ injury as subsequent hypotension.59
Continuous blood pressure monitoring detects more hypotension than measurements at 5-minute intervals,60,61 thus allowing clinicians to intervene earlier.61 An exciting recent development is an algorithm that predicts future hypotension from the arterial waveform.62 Although a small trial reported less hypotension when management was guided by the index,63 a larger one did not identify benefit.64 The difference likely results from differences in the treatment algorithms, and a robust trial is clearly needed.
Vasopressors like phenylephrine or norepinephrine are commonly used to treat hypotension during surgery. Phenylephrine is by far the most commonly used pressor in the United States,65 whereas norepinephrine is generally preferred elsewhere. Phenylephrine is a pure alpha agonist which raises blood pressure by increasing systemic vascular resistance, usually with a compensatory decrease in cardiac output.66 In contrast, norepinephrine combines powerful a-adrenergic agonism with weak b-adrenergic agonist activity which helps maintain cardiac output. Consequently, while blood pressure is comparably maintained with each vasopressor,67 phenylephrine reduces splanchnic blood flow and oxygen delivery.68 Clinicians should avoid phenylephrine in patients with septic shock.69
Despite the theoretical advantages of preserved cardiac output and splanchnic perfusion with the use of norepinephrine, there is limited evidence of any outcome improvement in surgical patients.70 Consequently both phenylephrine and norepinephrine are widely used in clinical practice, mostly based on clinical preference and availability. There is no convincing evidence that intraoperative low-dose vasopressors are themselves harmful, and allowing hypotension in an effort to avoid using vasopressors is probably unwise. Norepinephrine can be safely given through a central catheter or peripherally.71 In a recent study of 14,328 patients, there were only 5 extravasation events and not a single patient experienced local tissue injury.72
General hospital floor hypotension is common, prolonged, and profound. It is likely that most perioperative hypotensive organ injury occurs postoperative rather than intraoperatively. The challenge is that blood pressure is usually measured intermittently. Even at 4-hour intervals, about half of all potentially serious hypotensive episodes are missed.73 (Most hypoxemia is similarly missed with intermittent ward monitoring.30) Reliably detecting and treating ward hypotension will require continuous vital sign monitoring. But in the meantime, avoiding angiotensin converting enzyme inhibitors and angiotensin receptor blocks on the day of surgery helps,22 as does restarting chronic antihypertensive medications only when clearly needed.
Association versus Causality
Intraoperative hypotension is common. Depending on the definition and population, a quarter or more of all surgical patients have mean arterial pressures < 65 mmHg during surgery. Hypotension is also common postoperatively, with only about half of potentially serious episodes being detected by routine vital signs at 4-hour intervals.73 Postoperative hypotension is often prolonged, and it seems likely that much—or even most—myocardial and renal injury develops postoperatively.
There is currently sparse evidence that the associations between hypotension and MINS and AKI are causal. But a small randomized trial (n=292) demonstrated that preventing intraoperative hypotension reduces the risk of major complications by 25%, which is biologically plausible.74 Two large trials should identify what fraction (if any) of the observed associations are causal: POISE-3 (n=10,000, NCT03505723) is nearly finished and GUARDIAN (n=6,250, NCT pending) is about to start.
Summary
Intraoperative and postoperative hypotension are associated with myocardial and renal injury. Associations are consistently reported from various populations using various thresholds and analytic methods, and persist after adjustment for known baseline factors. (The associations with baseline factors are far stronger than for hypotension, but hypotension differs in being modifiable.) Associations between hypotension and delirium are also reported, but the evidence remains weak.
There is currently little randomized data to characterize the extent to which observed associations might be causal. Large trials are in progress, but results will not be available for some time. The question, then, is how to manage blood pressure in the meantime.
Two factors deserve special consideration. The first is how likely is a causal relationship between hypotension and organ injury? Surely much of the observed associations result from unobserved confounding or are predictive rather than modifiable. But it also seems likely that at least a fraction is causal, and thus amenable to intervention. The second factor to consider is how difficult it is to keep intraoperative mean arterial pressure above 65 mmHg or some similar threshold? In general, it is not difficult (or expensive) to keep intraoperative blood pressure well above the apparent harm threshold. In many cases, simply moderating anesthetic administration and skillfully managing fluids is sufficient. In others low- or moderate-dose vasopressors will be needed. There is no convincing evidence that administration of low-dose vasopressors is harmful. Preventing postoperative hypotension is far more challenging, but one helpful approach is to delay restarting chronic antihypertensive medications until clearly necessary.
Blood pressure—specifically hypotension prevention—is a modifiable factor that may reduce cardiovascular complications. Pending the results of robust trials, reasonable efforts to avoid perioperative hypotension seem prudent.
Daniel I. Sessler, MD, is the Michael Cudahy Professor and Chair, Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA.
The author is a consultant for Edwards Lifesciences (Irvine, CA) and serves on advisory boards and has equity interests in Sensifree (Cupertino, CA) and Perceptive Medical (Newport Beach, CA).
References
- Li G, Warner M, Lang BH, et al. Epidemiology of anesthesia-related mortality in the United States, 1999-2005. Anesthesiology. 2009;110:759–765.
- Pearse RM, Moreno RP, Bauer P, et al. European Surgical Outcomes Study group for the Trials groups of the European Society of Intensive Care M, the European Society of A: mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–1065.
- Bartels K, Karhausen J, Clambey ET, et al. Perioperative organ injury. Anesthesiology. 2013;119:1474–1489.
- The Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators: association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304.
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation Study Investigators: association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019;191:E830–E837.
- Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373:2258–2269.
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. Epub 2018 Aug 25.
- The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators: association between complications and death within 30 days after noncardiac surgery. Can Med Assoc J. 2019;191:E830–E837.
- Beattie WS, Wijeysundera DN, Chan MTV, et al. Anzca Clinical Trials Network for the ENIGMA-II Investigators: implication of major adverse postoperative events and myocardial injury on disability and survival: a planned subanalysis of the ENIGMA-II trial. Anesth Analg. 2018;127:1118–1126.
- Eikelboom JW, Connolly SJ, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330.
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391:2325–2334.
- Writing Committee for the Vision Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317:1642–1651.
- Duceppe E, Borges FK, Tiboni M, et al. Association between high-sensitivity troponin I and major cardiovascular events after non-cardiac surgery (abstrract). J Am Coll Cardiol. 2020;75.
- Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847.
- Myles PS, Leslie K, Chan MT, et al. Anzca Trials Group for the ENIGMA-II investigators: the safety of addition of nitrous oxide to general anaesthesia in at-risk patients having major non-cardiac surgery (ENIGMA-II): a randomised, single-blind trial. Lancet. 2014;384:1446–1454.
- Devereaux PJ, Sessler DI, Leslie K, et al. Poise-2 Investigators: clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1504–1513.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Poise-2 Investigators: aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503.
- Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126:47–65.
- Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123:79–91.
- Sessler DI, Meyhoff CS, Zimmerman NM, et al. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE-2 trial. Anesthesiology. 2018;128:317–327.
- Liem VGB, Hoeks SE, Mol K, et al. Postoperative hypotension after noncardiac surgery and the association with myocardial injury. Anesthesiology. 2020;133:510–522.
- Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126:16–27.
- Bijker JB, Gelb AW. Review article: The role of hypotension in perioperative stroke. Can J Anaesth. 2013;60:159–167.
- Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: A nested case-control study. Anesthesiology. 2012;116:658–664.
- Sun LY, Chung AM, Farkouh ME, et al. Defining an intraoperative hypotension threshold in association with stroke in cardiac surgery. Anesthesiology. 2018;129:440–447.
- Hsieh JK, Dalton JE, Yang D, et al. The association between mild intraoperative hypotension and stroke in general surgery patients. Anesth Analg. 2016;123:933–939.
- Turan A, Cohen B, Rivas E, et al. Association between postoperative haemoglobin and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Br J Anaesth. 2021;126:94–101.
- Turan A, Rivas E, Devereaux PJ, et al. Association between postoperative haemoglobin concentrations and composite of non-fatal myocardial infarction and all-cause mortality in noncardiac surgical patients: post hoc analysis of the POISE-2 trial. Br J Anaesth. 2021;126:87–93.
- Ruetzler K, Yilmaz HO, Turan A, et al. Intra-operative tachycardia is not associated with a composite of myocardial injury and mortality after noncardiac surgery: A retrospective cohort analysis. Eur J Anaesthesiol. 2019;36:105–113.
- Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg. 2015;121:709–715.
- Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110:505–515.
- Walsh M, Garg AX, Devereaux PJ, et al. The association between perioperative hemoglobin and acute kidney injury in patients having noncardiac surgery. Anesth Analg. 2013;117:924–931.
- Whitlock EL, Braehler MR, Kaplan JA, et al. Derivation, validation, sustained performance, and clinical impact of an electronic medical record-based perioperative delirium risk stratification tool. Anesth Analg. 2020;131:1901–1910.
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–515.
- Sun LY, Wijeysundera DN, Tait GA, et al. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523.
- O’Connor ME, Hewson RW, Kirwan CJ, et al. Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br J Surg. 2017;104:868–876.
- Turan A, Cohen B, Adegboye J, et al. Mild acute kidney injury after noncardiac surgery is associated with long-term renal dysfunction: a retrospective cohort study. Anesthesiology. 2020;132:1053–1061.
- Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116:987–997.
- Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50:206–217.
- Royse CF, Saager L, Whitlock R, et al. Impact of methylprednisolone on postoperative quality of recovery and delirium in the Steroids in Cardiac Surgery Trial: a randomized, double-blind, placebo-controlled substudy. Anesthesiology. 2017;126:223–233.
- Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology. 2009;111:1075–1084.
- Turan A, Duncan A, Leung S, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet. 2020;396:177–185.
- Gou RY, Hshieh TT, Marcantonio ER, et al. One-year medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surg. 2021;156:430–442.
- Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive care unit delirium: a review of diagnosis, prevention, and treatment. Anesthesiology. 2016;125:1229–1241.
- Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131:477–491.
- Pan H, Liu C, Ma X, et al. Perioperative dexmedetomidine reduces delirium in elderly patients after non-cardiac surgery: a systematic review and meta-analysis of randomized-controlled trials. Can J Anaesth. 2019;66:1489–1500.
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013;41:464–471.
- Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147:483–489.
- Feng X, Hu J, Hua F, et al. The correlation of intraoperative hypotension and postoperative cognitive impairment: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2020;20:193.
- Maheshwari K, Ahuja S, Khanna AK, et al. Association between perioperative hypotension and delirium in postoperative critically ill patients: a retrospective cohort analysis. Anesth Analg. 2020;130:636–643.
- Hori D, Brown C, Ono M, et al. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br J Anaesth. 2014;113:1009–1017.
- Hirsch J, DePalma G, Tsai TT, et al. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br J Anaesth. 2015;115:418–426.
- Wesselink EM, Kappen TH, van Klei WA, et al. Intraoperative hypotension and delirium after on-pump cardiac surgery. Br J Anaesth. 2015;115:427–433.
- Langer T, Santini A, Zadek F, et al. Intraoperative hypotension is not associated with postoperative cognitive dysfunction in elderly patients undergoing general anesthesia for surgery: results of a randomized controlled pilot trial. J Clin Anesth. 2019;52:111–118.
- Brown CH 4th, Neufeld KJ, Tian J, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: A nested randomized clinical trial. JAMA Surg. 2019;154:819–826.
- Brown CH 4th, Probert J, Healy R, et al. Cognitive decline after delirium in patients undergoing cardiac surgery. Anesthesiology. 2018;129:406–416.
- Mrkobrada M, Chan MTV, Cowan D, et al. Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019; 394:1022–1029.
- Sessler DI, Khan MZ, Maheshwari K, et al. Blood pressure management by anesthesia professionals: evaluating clinician skill from electronic medical records. Anesth Analg. 2021;132:946–956.
- Maheshwari K, Turan A, Mao G, et al. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73:1223–1228.
- Naylor AJ, Sessler DI, Maheshwari K, et al. Arterial catheters for early detection and treatment of hypotension during major noncardiac surgery: a randomized trial. Anesth Analg. 2020;131:1540–1550.
- Maheshwari K, Khanna S, Bajracharya GR, et al. A randomized trial of continuous noninvasive blood pressure monitoring during noncardiac surgery. Anesth Analg. 2018;127:424–431.
- Davies SJ, Vistisen ST, Jian Z, et al. Ability of an arterial waveform analysis-derived hypotension prediction index to predict future hypotensive events in surgical patients. Anesth Analg. 2020;130:352–329.
- Wijnberge M, Geerts BF, Hol L, et al. Effect of a machine learning-derived early warning system for intraoperative hypotension vs standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: the HYPE randomized clinical trial. JAMA. 2020;323:1052–1060.
- Maheshwari K, Shimada T, Yang D, et al. Hypotension prediction index for prevention of hypotension during moderate- to high-risk noncardiac surgery. Anesthesiology. 2020;133:1214–1222.
- Farag E, Makarova N, Argalious M, et al. Vasopressor infusion during prone spine surgery and acute renal injury: a retrospective cohort analysis. Anesth Analg. 2019;129:896–904.
- Ducrocq N, Kimmoun A, Furmaniuk A, et al. Comparison of equipressor doses of norepinephrine, epinephrine, and phenylephrine on septic myocardial dysfunction. Anesthesiology. 2012;116:1083–1091.
- Morelli A, Ertmer C, Rehberg S, et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Crit Care. 2008;12:R143.
- Reinelt H, Radermacher P, Kiefer P, et al. Impact of exogenous beta-adrenergic receptor stimulation on hepatosplanchnic oxygen kinetics and metabolic activity in septic shock. Crit Care Med. 1999;27:325–331.
- Dellinger RP, Levy MM, Carlet JM, et al. International Surviving Sepsis Campaign Guidelines C, American Association of Critical-Care N, American College of Chest P, American College of Emergency P, Canadian Critical Care S, European Society of Clinical M, Infectious D, European Society of Intensive Care M, European Respiratory S, International Sepsis F, Japanese Association for Acute M, Japanese Society of Intensive Care M, Society of Critical Care M, Society of Hospital M, Surgical Infection S, World Federation of Societies of I, Critical Care M: Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
- Mets B: Should norepinephrine, rather than phenylephrine, be considered the primary vasopressor in anesthetic practice? Anesth Analg. 2016;122:1707–1714.
- Owen VS, Rosgen BK, Cherak SJ, et al. Adverse events associated with administration of vasopressor medications through a peripheral intravenous catheter: a systematic review and meta-analysis. Crit Care. 2021;25:146.
- Pancaro C, Shah N, Pasma W, et al. Risk of major complications after perioperative norepinephrine infusion through peripheral intravenous lines in a multicenter study. Anesth Analg. 2019;131:1060–1065.
- Turan A, Chang C, Cohen B, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology. 2019;130:550–559.
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318:1346–1357.