INTRODUCTION
Mpox, previously known as Monkeypox, is a global health concern.1 While first detected in humans in 1970 in the Republic of the Congo, its spread to nonendemic countries in 2022 led the World Health Organization (WHO) to establish emergency measures to mitigate development of a pandemic. As of November 7, 2022, the WHO has reported 78,474 confirmed cases and 3,685 probable cases within over 109 countries. The United States is the most affected country with 28,651 reported cases.2 The preferred name for this virus was changed by the WHO to Mpox in November of 2022. Health care providers are likely to encounter confirmed and/or suspected Mpox cases in the perioperative arena.
As leaders in patient safety, anesthesia professionals have an opportunity to leverage current evidence and create systems of care to improve the perioperative safety of patients through infection prevention. In this brief review, we provide a pragmatic framework for the perioperative care of the patient infected with Mpox by drawing on infection prevention and control principles and measures. We focus on pragmatic considerations based on the current literature, professional society statements, and current knowledge on the management of infection control of enveloped viruses in the perioperative environment.
Mpox, an enveloped, double-stranded DNA virus, is a member of the Poxviridae family and orthopoxvirus genus.3 Two distinct viral subtypes include the Congo Basin and West African strains. While the West African subtype is the dominant strain worldwide,4,5 with an estimated mortality rate of 1%,6 the less dominant Congo Basin strain is reported to transmit more easily between humans and is associated with up to a 10% mortality rate.7 Complications of Mpox can include secondary infections, bronchopneumonia, sepsis, encephalitis, and infection of the cornea with ensuing vision loss. Nosocomial transmission is rare, but it has been reported to occur through direct contact with affected skin or environmental surfaces and/or via respiratory droplets. These modes of transmission provide some urgency for anesthesia professionals to prepare for infection prevention in the anesthesia work environment.
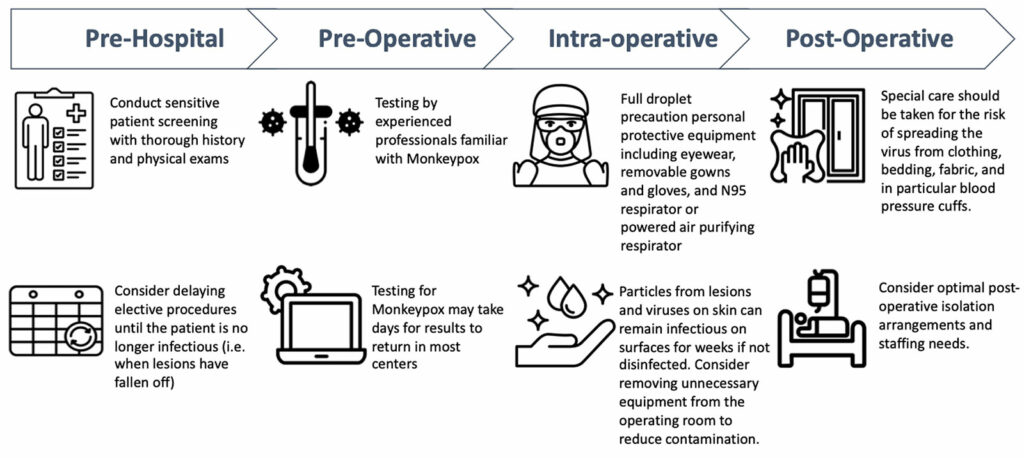
The WHO issued guidelines for the clinical management and infection prevention and control for Mpox in June 2022.8 General recommendations included contact and droplet precautions for any confirmed patient and the use of respirators and airborne precautions for aerosol-generating procedures. The American Society of Anesthesiologists and the Anesthesia Patient Safety Foundation provided a joint statement of support and recommendations on August 31, 2022.9 Based on these guidelines, a pragmatic framework for the preparation and optimal care of patients with Mpox specific to the anesthesia work environment was developed (Figure 1). Important considerations include preoperative screening and testing, decision-making considerations as to proceed with or to delay elective surgery, and intra- and postoperative infection control measures.
SCREENING AND ELECTIVE SURGERY CONSIDERATIONS
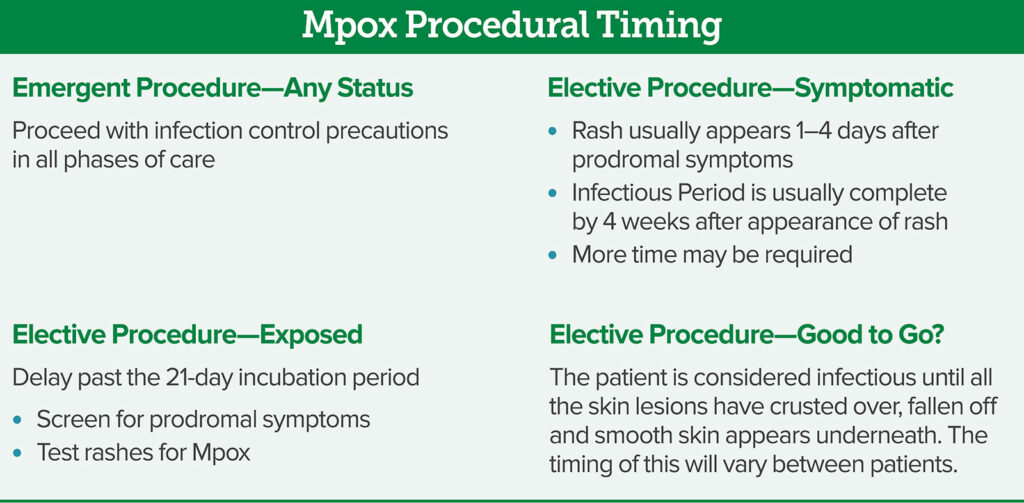
Ideally, adult and pediatric patients with Mpox, or Mpox exposure, will be identified pre-operatively. In general, persons are considered exposed after direct contact with the skin lesions or bodily fluids of an infected individual or indirect contact through objects that contact skin lesions or bodily fluids (e.g., bed linens). Infected individuals may report a variety of constitutional symptoms including fever, malaise, weakness, lymphadenopathy,10,11 and rash that may take 4 weeks to resolve; although some may present with minimal or no symptoms. Mpox infection is accompanied by skin lesions that may be widespread or limited to a few lesions. The lesions, often described as painful, often occur in the genital or anorectal areas, which can make screening potentially challenging. Thus, patients who report being exposed to Mpox or diagnosed with Mpox should have elective surgery deferred until there is no concern for transmission9 (Figure 2).
The purpose of delaying an elective surgery is to reduce the risk of Mpox transmission. Defining the duration of an infectious period of Mpox can be challenging. The variable incubation period and the many weeks the rash may take to resolve make it difficult to estimate. This includes delaying an elective procedure for at least 21 days from the exposure, given the reported incubation period ranging from 4 to 21 days,12 or, in the case of a rash, up to 4 weeks. The patient with Mpox-associated rash is no longer considered infectious when lesions have fallen off and are replaced with smooth skin. It is reasonable that patients that have active lesions and rashes consistent with Mpox not undergo elective surgery.
Importantly, if a concern for Mpox arises during the pre-operative exam, the interview should be interrupted, and appropriate personal protective equipment (PPE) donned. Care must be taken by health care professionals to not attach social stigmas with Mpox infections. Complaints of unexplained rectal or genital pain and/or perioral pustules or sores should prompt consideration for Mpox exposure or risk factors (Table 1). Integration of screening tools into electronic health record systems may facilitate perioperative screening and communication of patient risks.13,14
Table 1: Clinical Questions to Help Guide Screening and Perioperative Decision-Making With Patients Suspected to Have Mpox.
| Perioperative Clinical Questions: |
|
MPOX TESTING
Information on current recommendations is available on the Centers for Disease Control and Prevention website (https://www.cdc.gov/poxvirus/mpox/clinicians/index.html). Currently, routine testing for Mpox is not recommended. Mpox can be detected using polymerase chain reaction assays from DNA sampled from lesions. Blood testing is not recommended as Mpox virus remains in the blood for only a short period of time. Results from testing for Mpox may require days to return. If concern for Mpox develops intraoperatively, we recommend contacting your local infection control officer or infectious disease specialist as soon as possible to discuss how to prevent further exposures and inform health care workers who may have already been exposed. Postexposure vaccination for prophylaxis is available and requires utilization within 4 days of exposure to optimize prevention of disease. Vaccination between 4 and 14 days following the date of exposure is reasonable to consider, but less effective.15
OPERATING ROOM CONSIDERATIONS
Mpox is a large virus that is spread primarily cles from lesions and viruses on the skin can remain infectious on surfaces for extended durations without disinfection. For example, one study detected viable Mpox on a household surface 15 days after the infected individual left the home.16 There is a risk of spreading the virus when clothing, bedding, or other fabric is moved. Caution should be taken in moving fabrics that have been in contact with the patient. Mpox virus has been isolated in samples obtained from the air during bed linen changes. Further caution includes monitors such as the blood pressure cuff. For example, care should be taken to avoid frequent and rapid removal of the blood pressure cuff as the process of removal can spread the virus. All fabric that has contacted the patient should be discarded in sealed waste bags to prevent aerosolization of viral particles.
Patients should undergo care in negative pressure rooms for aerosol generating procedures. Health care workers should have full droplet precaution PPE when caring for patients with Mpox. An N95 respirator or powered air purifying respirator (PAPR) is recommended. Protective eyewear is required, as is a removable protective gown and gloves. Unnecessary equipment should be removed from the operating room (OR), OR traffic should be limited, and multiple anesthetics in the same OR should be avoided. Evidence-based anesthesia work area infection control measures should be followed, including frequent hand hygiene and postinduction environmental cleaning.17,18
Mpox, an enveloped virus, is effectively inactivated via use of United States Environmental Protection Agency (EPA)-registered disinfectants. Examples of EPA-registered disinfectants that can be used for Mpox include cleaning solutions that have the active ingredients of isopropyl alcohol, quaternary ammonium, or ethyl alcohol. A comprehensive list of recommended products for disinfection can be found at the EPA website (https://www.epa.gov/pesticide-registration/disinfectants-emerging-viral-pathogens-evps-list-q).19
POSTOPERATIVE
An important consideration in the postoperative period is to attempt to minimize the transport and movement of infected patients and exposed health care providers across the health care system. Expedited dismissal from the OR should be considered when applicable. The surgical emergencies that may prompt patients with active Mpox to undergo surgery often require postoperative hospitalization or intensive care. Full PPE and isolation will need to be utilized in the care of these patients for transport and recovery. Health care workers that have unprotected exposure to patients with Mpox may require isolation for up to three weeks with those who develop lesions isolated until they are no longer infectious.9
CONCLUSION
Patients presenting with Mpox and/or exposure present unique perioperative considerations. Anesthesia care team providers can leverage current knowledge and pragmatic approaches for infection prevention and control to optimize perioperative patient and provider safety.
References
- World Health Organization. WHO recommends new name for monkeypox disease. Accessed Dec 1, 2022, https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease.
- World Health Organization. 2022 monkeypox outbreak: global trends. Updated November 8, 2022. Accessed November 8, 2022. https://worldhealthorg.shinyapps.io/mpx_global/.
- Tiecco G, Degli Antoni M, Storti S, et al. Monkeypox, a literature review: what is new and where does this concerning virus come from? Viruses. 2022;14:1894.
- Hutson CL, Abel JA, Carroll DS, et al. Comparison of West African and Congo Basin monkeypox viruses in BALB/c and C57BL/6 mice. PLoS One. 2010;5:e8912.
- Forni D, Molteni C, Cagliani R, Sironi M. Geographic structuring and divergence time frame of monkeypox virus in the endemic region. J Infect Dis. 2022;Jul 14;jiac298. Online ahead of print.
- Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target Ther. 2022;7:373.
- McCarthy MW. Recent advances in the diagnosis monkeypox: implications for public health. Expert Rev Mol Diagn. 2022;22:739–744.
- World Health Organization. Clinical management and infection prevention and control for monkeypox: interim rapid response guidance. Accessed November 29, 2022, https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1
- American Society of Anesthesiologists. ASA/APSF Statement on Monkeypox. Accessed November 29, 2022, https://www.asahq.org/about-asa/newsroom/news-releases/2022/08/asa-apsf–statement-on-monkeypox.
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28–34.
- Ranganath N, Tosh PK, O’Horo J, et al. Monkeypox 2022: gearing up for another potential public health crisis. Mayo Clin Proc. 2022;97:1694–1699.
- Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:1257.
- Birkhead GS, Klompas M, Shah NR. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 2015;36:345–59.
- van den Blink A, Janssen LMJ, Hermanides J, et al. Evaluation of electronic screening in the preoperative process. J Clin Anesth. 2022;82:110941.
- Centers for Disease Control and Prevention. Mpox vaccine considerations. Accessed Nov 29, 2022, https://www.cdc.gov/poxvirus/monkeypox/clinicians/vaccines/vaccine-considerations.html.
- Morgan CN, Whitehill F, Doty JB, et al. Environmental persistence of Monkeypox virus on surfaces in household of person with travel-associated infection, Dallas, Texas, USA, 2021. Emerg Infect Dis. 2022;28:1982–1989.
- Loftus RW, Dexter F, Goodheart MJ, et al. The effect of improving basic preventive measures in the perioperative arena on staphylococcus aureus transmission and surgical site infections: a randomized clinical trial. JAMA Netw Open. 2020 Mar 2;3(3):e201934.
- Agency for Healthcare Research and Quality. Central line-associated bloodstream infections (CLABSI). Accessed July 26, 2022, https://www.ahrq.gov/topics/central-line-associated-bloodstream-infections-clabsi.html.
- United States Environmental Protection Agency. Disinfectants for emerging viral pathogens (EVPs): List Q. Accessed Nov 29, 2022, https://www.epa.gov/pesticide-registration/disinfectants-emerging-viral-pathogens-evps-list-q.


Leave a Reply
You must be logged in to post a comment.