It is not uncommon for patients to ask if and how anesthesia will affect their brain. Perioperative bain health is a particular concern for older patients, families, and caregivers. As such, brain health has been recognized as an APSF Patient Safety Priority. The number of Americans aged 65 and older is predicted to double to 95 million by 2060,1 and nearly 40% of all surgical procedures are performed on patients over 65.2 With age, comorbidities increase in frequency and complexity, challenging perioperative care and contributing to their risk of worse outcomes, including perioperative neurocognitive disorders (PND).1 Optimizing brain health with interventions in the perioperative period is of paramount importance. Anesthesia professionals, as integral members of the perioperative team, are uniquely positioned to improve patient outcomes by identifying patients at risk of PND and ensuring specific steps are taken to reduce its occurrence.
Multiple societies and organizations have proposed recommendations, outlined frameworks, and published guidelines for perioperative brain health.3-8 Following these recommendations, many health care institutions have established programs to prevent PND in surgical patients. These guidelines and programs all highlight the need for a multidisciplinary team-based approach with interventions in the preoperative, intraoperative, and postoperative periods.
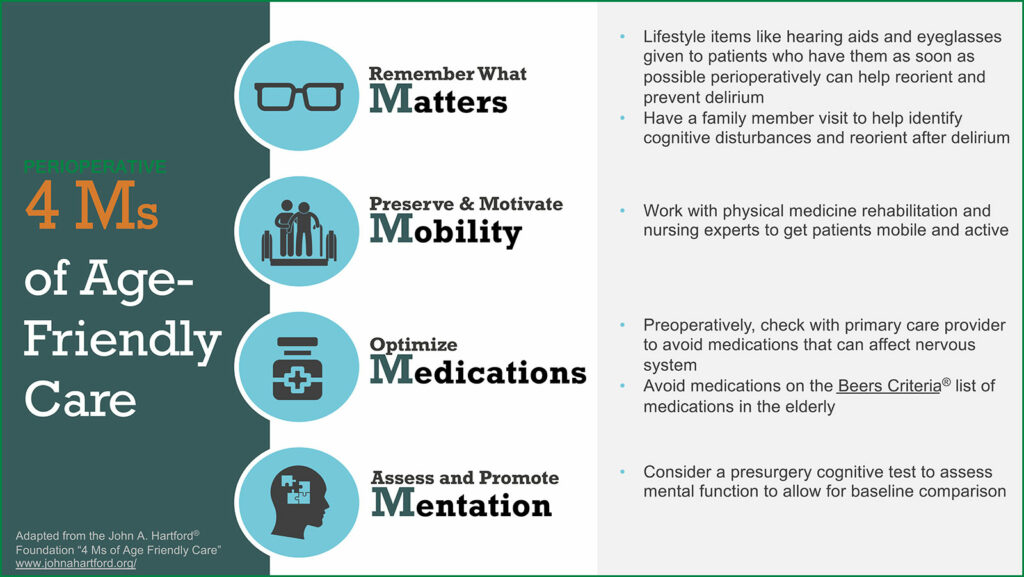
The National Academy of Medicine has recognized the increasing population of elderly patients as a defining challenge of the 21st century.9 As such, in 2017 The John A. Hartford Foundation and the Institute of Healthcare Improvement, in partnership with the American Hospital Association and the Catholic Health Association of the United States, launched the “Age-Friendly Health System” to improve the health, productivity, and quality of life of older adults.
The “Age-Friendly Health System” uses the framework of the 4 Ms: What Matters, Mobility, Medication, and Mentation (Figure 1).10
IMPACT OF PERIOPERATIVE NEUROCOGNITIVE DISORDERS (PND)
Postoperative delirium, characterized by inattention and confusion occurring within seven days of surgery, is the most common adverse event after surgery in older adults with an incidence of up to 65%.3 Health care costs increase with postoperative delirium, with an estimated toll of $32.9 billion per year.11 More is known about the factors contributing to postoperative delirium than the other perioperative neurocognitive disorders. When predisposing factors such as age >65, pre-existing cognitive decline, poor baseline functional status, visual or sensory impairment, and chronic illness are combined with precipitating factors such as duration and invasiveness of surgery, postoperative pain management, and use of certain medications, the risk for postoperative delirium is increased. In addition, postoperative delirium is associated with increased length of stay, higher morbidity and mortality, and severe distress to patients and their family members.4,12 Patients with normal preoperative cognition who experience postoperative delirium are more likely to develop cognitive impairment later.13,14 Delirium has also been shown to be associated with longer-term neurocognitive decline.3,15 The Hospital Elder Life Program (HELP), an evidence-based approach targeted at risk factors for delirium showed that almost half of delirium cases could be prevented.16 In a study of a modified HELP protocol in surgical patients (orienting communication, early mobilization, and oral and nutritional assistance), the incidence of delirium decreased by 56%. The authors of this study credited the program’s effectiveness to daily adherence to the protocol, facilitated by dedicated nurses. Several centers have now published their experiences and results with implementation of these guidelines, with evidence that delirium can be prevented.17
WHAT CAN ANESTHESIA PROFESSIONALS DO?
Several professional societies have published best practice guidelines for maintaining perioperative brain health. The American Geriatrics Society (AGS),7 the American College of Surgeons (ACS),18 the American Society of Anesthesiologists’ Brain Health Initiative (ASA),4 as well as the Sixth Perioperative Quality Initiative consensus conference (POQI-6) and the Fifth International Perioperative Neurotoxicity Working Group5 have recommendations to guide health care professionals in identifying patients at risk of cognitive decline and preventing cognitive impairment after surgery. Preexisting cognitive impairment is a significant risk factor for postoperative delirium and other complications.19,6 All of these guidelines recommend that cognitive screening and an assessment of risk factors for PND should be conducted for all patients over 65.4-8 Several cognitive screening tools, such as the Mini-Cog, the Mini-Mental State Examination (MMSE), and the Montreal Cognitive Assessment (MoCA) are quick, easy to use, require no formal training, and could be applied in the preoperative clinic.1,6 With the identification of an abnormal screening test, patients can receive further evaluation and treatment for a potential cognitive deficit, be informed of the risk of PND prior to surgical intervention, and be referred to resources and interventions beneficial to high-risk patients.1,6 Interventions for delirium include mobilization, orientation, sleep hygiene, returning personal items (glasses, hearing aids and dentures) after surgery, and education about delirium for health care professionals.4-8
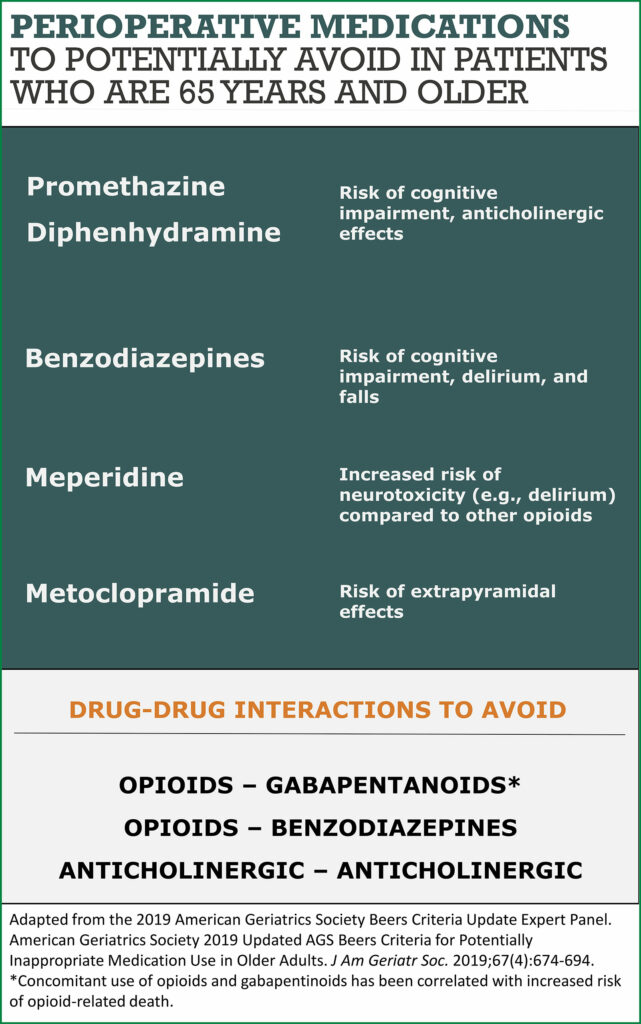
There is also evidence supporting the avoidance of specific medications in patients at risk of PND (Figure 2). The American Geriatrics Society Beers Criteria recommends avoiding potentially inappropriate medications such as benzodiazepines, anticholinergics, antipsychotics, meperidine, and gabapentin in high-risk patients.20 A multimodal regimen with limited opioids is recommended.21 Strong evidence supporting the association between these medications and postoperative delirium makes these recommendations an important potential target for improving perioperative brain health.15
While there is agreement in the above recommendations, other areas remain uncertain. Data are conflicting regarding the use of processed electroencephalogram (EEG)-guided anesthetic dosing to decrease postoperative delirium and PND; however, some authors argue that there may be a subset of cognitively frail patients who could benefit from EEG-guided avoidance of anesthetic overdose resulting in brain activity suppression.1 Similarly, there are conflicting data regarding the impact of intraoperative blood pressure management and choice of anesthetic technique on PND. The Best Practices for Perioperative Brain Health state that while further research is warranted in these areas, anesthesia professionals “should monitor age-adjusted end-tidal minimal alveolar concentration (MAC) fraction, strive to optimize cerebral perfusion, and perform EEG-based anesthetic management in older adults.”6
Comprehensive programs to identify patients at risk and address multiple factors contributing to perioperative brain health are necessary. Authors at the University of California, San Francisco have described their experience with implementing a “Perioperative Delirium Prevention and Treatment Pathway” for perioperative brain health.15,22 First, they identified stakeholders and received their feedback. They then provided educational material through meetings and email. In their pathway, patients were screened with the Age, WORLD backwards, Orientation, iLlness severity, Surgery-specific risk (AWOL-S) tool: Age>80, failure to spell “World” backward, disorientation to place, ASA status, and a surgery-specific risk based on National Surgical Quality Improvement Program (NSQIP) data. Patients with a greater than 5% risk for delirium were flagged in the electronic medical record (EMR) with a banner. To ease implementation, the delirium screening questions were embedded into the existing questions asked by the preoperative nurses. The standard PACU order set, which includes several of the Beers Criteria Potentially Inappropriate Medications (PIMs), was modified to omit these medications. Delirium risk was also added to the standard PACU handoff tool. The authors emphasized that changes integrated into existing workflows and automated processes through the EMR were most successful in promoting changes in behavior.22
Implementing routine cognitive screening at the preoperative evaluation clinic at the University of Southern California revealed that preoperative cognitive screening with the Mini-Cog test was feasible without prior experience in cognitive screening. High-risk patients were flagged with alerts in the EMR and referred to a geriatrician and geriatric pharmacist before surgery. They found that 21% of their patients screened positive for cognitive impairment and that a significant proportion of patients would have been missed without a formal cognitive screen. These findings increased “buy-in” at their preoperative clinic and in their institution.23
As research continues to answer many remaining questions, how can we integrate the existing recommendations and published experience into our clinical practice? Despite recent recommendations on perioperative brain health and a call to action by the ASA’s Brain Health Initiative,4 a recent survey reported that preoperative screening occurred in less than 10% of cases.24 Several authors have emphasized the importance of engaging the many stakeholders including nurses, surgeons, patients, families, organizational and departmental leadership, and pharmacists.15,23 Preexisting Enhanced Recovery After Surgery (ERAS) protocols, which use a multidisciplinary team-based approach to improve various aspects of perioperative care with evidence-based interventions, could be used to help implement perioperative brain health recommendations.25 Since its inception in 2005, ERAS has expanded worldwide and is now widely accepted within the field of perioperative medicine. Researchers have proposed a “Brain-ERAS” protocol that, rather than being a separate protocol, is incorporated into existing ERAS protocols.25
Given the wide availability of information technology, more patients are taking steps to be informed and active participants in their health. Anesthesia professionals should take advantage of this movement and help patients, their caregivers, and their care teams optimize patient outcomes, including preventing PND in those at risk.
REFERENCES
- Vacas S, Canales C, Deiner SG, Cole DJ. Perioperative health in the older adult: a patient safety imperative. Anesth Analg. 2022;135:316–328. PMID: 35584550
- Centers for Disease Control and Prevention, “Number of Discharges from Short-Stay Hospitals, by First-Listed Diagnosis and Age: United States 2010,” https://www.cdc.gov/nchs/data/nhds/3firstlisted/2010first3_numberage.pdf. Accessed October 30, 2022.
- Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, et al. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth. 2019;123:464–478. PMID: 31439308
- Peden CJ, Miller TR, Deiner SG, et al. Improving perioperative brain health: an expert consensus review of key actions for the perioperative care team. Br J Anaesth. 2021;126:423–432. PMID: 33413977
- Hughes CG, Boncyk CS, Culley DJ, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020;130:1572–1590. PMID: 32022748
- Berger M, Schenning KJ, Brown CH, et al. Best practices for postoperative brain health: recommendations from the Fifth International Perioperative Neurotoxicity Working Group. Anesth Analg. 2018;127:1406–1413. PMID: 30303868
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136–148.e1. PMID: 25535170
- Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192–214. PMID: 28187050
- Dzau VJ, Inouye SK, Rowe JW, et al. Enabling healthful aging for all—The National Academy of Medicine grand challenge in healthy longevity. N Engl J Med. 2019;381:1699–1701. PMID: 31633895
- The John A. Hartford® Foundation, “4 Ms of Age-Friendly Care.” https://www.johnahartford.org/grants-strategy/current-strategies/age-friendly/age-friendly-care. Accessed November 7, 2022.
- Gou RY, Hshieh TT, Marcantonio ER, et al. One-year medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surg. 2021;156:430–442. PMID: 33625501
- Partridge JSL, Crichton S, Biswell E, et al. Measuring the distress related to delirium in older surgical patients and their relatives. Int J Geriatr Psychiatry. 2019;34:1070–1077. PMID: 30945343
- Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119:316–323. PMID: 28854531
- Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. 2020;77:1373–1381. PMID: 32658246
- Curtis MS, Forman NA, Donovan AL, Whitlock EL. Postoperative delirium: why, what, and how to confront it at your institution. Curr Opin Anaesthesiol. 2020;33:668–673. PMID: 32796170
- Hshieh TT, Yang T, Gartaganis SL, et al. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26:1015–1033. PMID: 30076080
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg. 2017;152:827–834. PMID: 28538964
- Mohanty S, Rosenthal RA, Russell MM, et al. Optimal perioperative management of the geriatric patient: a best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg. 2016;222:930–947. PMID: 27049783
- Culley DJ, Flaherty D, Fahey MC, et al. Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Anesthesiology. 2017;127:765–774. PMID: 28891828
- The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–694. PMID: 30693946
- Wilson SH, Wilson PR, Bridges KH, et al. Nonopioid analgesics for the perioperative geriatric patient: a narrative review. Anesth Analg. 2022;135:290–306. PMID: 35202007
- Donovan AL, Braehler MR, Robinowitz DL, et al. An implementation-effectiveness study of a perioperative delirium prevention initiative for older adults. Anesth Analg. 2020;131:1911–1922. PMID: 33105281
- Decker J, Kaloostian CL, Gurvich T, et al. Beyond cognitive screening: establishing an interprofessional perioperative brain health initiative. J Am Geriatr Soc. 2020;68:2359–2364. PMID: 32748487
- Deiner S, Fleisher LA, Leung JM, et al. Adherence to recommended practices for perioperative anesthesia care for older adults among US anesthesiologists: results from the ASA Committee on Geriatric Anesthesia-Perioperative Brain Health Initiative ASA member survey. Perioper Med (Lond). 2020;9:6. PMID: 32123562
- Safavynia SA, Goldstein PA, Evered LA. Mitigation of perioperative neurocognitive disorders: a holistic approach. Front Aging Neurosci. 2022;14:949148.


Leave a Reply
You must be logged in to post a comment.