The benefits of low-flow anesthesia are well established and include reduced inhaled anesthetic waste, decreased cost, and fewer greenhouse gas effects.1 For the individual patient, low-flow anesthesia reduces loss of heat and humidity from the lungs.2 This article will highlight the common safety concerns of low-flow anesthesia. This is not intended to be a comprehensive guide to practicing low-flow anesthesia, which is well-described in the literature,3 and is a topic that will be covered in the upcoming APSF-ASA medical technology training initiative. The good news is that the risks of adopting low-flow anesthesia are readily managed, and patient safety concerns should not be a barrier to reducing fresh gas flow.
The “circle system” was designed to reduce anesthetic waste by allowing exhaled anesthetic agent to return to the patient in the inspired gases (Figure 1). Carbon dioxide (CO2) absorption is fundamental to the design of the circle system. While CO2 absorbents are necessary for safe use of the circle system, the presence of an absorbent does not guarantee the circle system is actually reducing waste. Effectively reducing waste requires the anesthesia professional to reduce fresh gas flow in a manner that allows exhaled gases to return to the patient.4
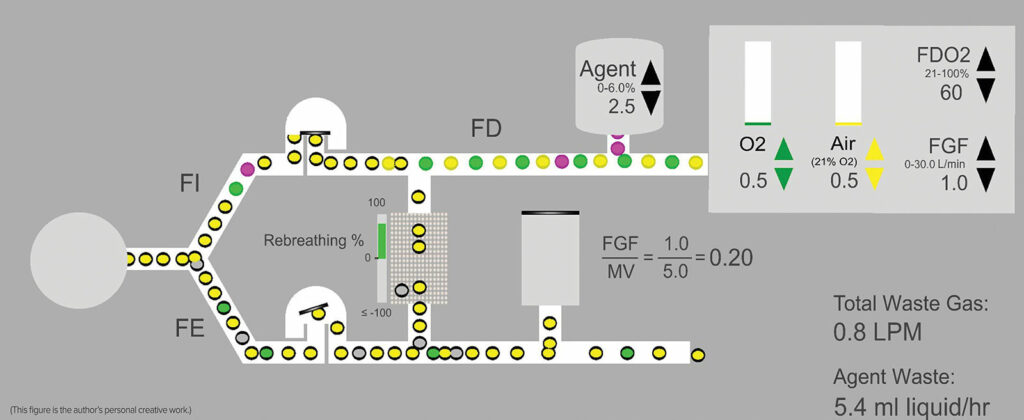
Figure 1: Idealized schematic of a circle system where FGF is a fraction of the minute ventilation at 1 L/min—0.5 L/min Air and O2 respectively. Air = Yellow circles, Oxygen = Green circles, and Agent = purple circles. Circles with the black border = exhaled gases or anesthetic, some of which return to the inspired limb. Note that due to recirculation of exhaled gases, the concentration of oxygen and anesthetic delivered in the fresh gas flow (FDO2 60% and FDA 2.5%) will be different from the inspired concentrations (FIO2 and FIA) due to mixing of fresh gas with exhaled gases (FEO2 and FEA). The exact concentration differences will depend upon the phase of the procedure with the difference diminishing over time. FD = delivered fraction, FI = inspired fraction; FE = expired fraction; FGF = fresh gas flow; MV = minute ventilation.
Low-flow anesthesia is sometimes described as a total fresh gas flow of 1 liter/min. In practice however, low-flow anesthesia is not a single number. Depending upon the circumstances, 1 liter/min can be too much to achieve the desired degree of waste reduction, or too little to maintain an adequate concentration of oxygen or anesthetic in the circuit. For purposes of this discussion, the authors define the current practice of low-flow anesthesia as: Reducing fresh gas flow below minute ventilation to the lowest level consistent with equipment capabilities and provider comfort while ensuring safe and effective care for the patient. While reducing fresh gas flow unquestionably reduces waste, cost, and pollution, it is not without consequences that have implications for patient safety.
Effective oxygen delivery requires an inspired oxygen concentration that will maintain the desired concentration of oxygen in the blood. Anesthetic agent requirements are dictated by the need to maintain an adequate level of hypnosis and physiologic stability in the face of surgical stimulation and trauma. As fresh gas flow is reduced and rebreathing increases, the concentrations delivered in the fresh gas and the concentrations inspired by the patient can be quite different. Furthermore, gas and agent concentrations change in the circuit more slowly as fresh gas flow is reduced. Managing the relationship between delivered and inspired concentrations is the art and practice of low-flow anesthesia. It is important to note that control of carbon dioxide concentration is determined by minute ventilation and is unaffected by fresh gas flow.
Ensuring Adequate Oxygen Delivery
Concern for inadequate oxygen delivery leading to hypoxemia or inadvertent low inspired concentration of oxygen is reasonable as fresh gas flow is reduced. The concentration of oxygen in the exhaled gas (FEO2) is always less than the inspired concentration (FIO2) due to the patient’s oxygen consumption. As the percentage of rebreathed gas increases, FEO2 mixes with the oxygen delivered to the patient in the fresh gas (FDO2) to yield the FIO2. The more exhaled gas is allowed to return to the patient, the greater the impact of FEO2 on FIO2 (Figure 1).
Continuous monitoring of inspired oxygen concentration is essential to the safe and effective practice of low-flow anesthesia. As flows are reduced, the practitioner estimates the delivered oxygen concentration (FDO2) that will maintain the desired inspired concentration (FIO2). Ultimately, the patient’s oxygen consumption and any leaks in the circuit will determine the FIO2 delivered to the patient.
Continuous FIO2 monitoring will help to guide adjustments to fresh gas flow. Since the FIO2 changes slowly at low fresh gas flow, a low oxygen concentration alarm can be set above the minimum safe level to provide a notification if FIO2 is heading lower than desired.
Managing inspired oxygen concentration during low-flow anesthesia is relatively straightforward since oxygen consumption is fairly constant during a procedure. Managing inspired anesthetic agent concentration is a bit more challenging since the uptake of agent falls exponentially over time.
Ensuring Adequate Inspired Anesthetic Agent Concentration
As mentioned previously, safe anesthetic agent delivery requires that the patient have a sufficient concentration to be unaware, but not so much that physiologic stability is threatened. Similar to the case of oxygen, the expired concentration of anesthetic agent (FEAgent) will always be less than the inspired concentration of agent (FIAgent) due to uptake, except during emergence. Early in the procedure, when uptake of agent is high, the difference between FEAgent and FIAgent can be substantial. For that reason, it is more difficult to reduce flows during induction and maintain the desired anesthetic concentration compared with the maintenance phase of the anesthetic when uptake has slowed and FEAgent approaches FIAgent.
Continuous monitoring of inspired and expired anesthetic agent concentration is essential to the safe and effective practice of low-flow anesthesia. The difference between inspired and expired anesthetic agent concentration indicates the rate of uptake. As the difference narrows, uptake is slowing and it is easier to reduce flows and maintain the desired anesthetic concentration in the circuit. While the Delivered agent concentration,FDAgent, is determined by the vaporizer setting, the FIAgent indicates what is being inspired by the patient. As flows are reduced, it may be necessary to increase the vaporizer setting above the Minimum Alveolar Concentration (MAC) concentration desired in the patient to maintain the FIAgent and FEAgent at the desired levels. Like oxygen delivery, setting the vaporizer is an estimate by the low-flow practitioner, and continuous agent concentration monitoring becomes essential to guiding vaporizer and fresh gas flow settings.
Managing Fresh Gas Flow when Changing Oxygen and Agent Concentrations
One major challenge to the practice of low-flow anesthesia is the rate of change of oxygen and agent concentrations in the circuit. The time constant for the rate of change is the internal volume of the anesthesia machine and breathing circuit in liters divided by the fresh gas flow in L/min. The internal volume can be 5 liters or more so that a fresh gas flow of 1 L/min could result in a time constant of 5 minutes, and it can take four-time constants to get close to equilibrium.
As fresh gas flow is reduced, concentrations of oxygen and anesthetic will change more slowly to reach a new equilibrium. As a result, the practitioner may change the gas mixture or vaporizer setting, but the ultimate impact on concentrations in the circuit will not be apparent for several minutes. This is another reason for continuous monitoring of oxygen and agent concentrations in the circuit as well as the use of high and low alarm limits to draw attention to slow changes that might otherwise go unnoticed. Indeed, it may be necessary to increase the total fresh gas flow to ensure that oxygen and agent concentrations change more quickly if needed.
Does Sevoflurane have a Minimum Safe Fresh Gas Flow?
The package insert for sevoflurane indicates that sevoflurane is safe when fresh gas flow is not less than 1 L/min for up to 2 MAC-Hours or not less than 2 L/min for longer procedures.5 This recommendation is neither scientifically sound nor consistent with a modern practice of low-flow anesthesia. Nevertheless, given the FDA labelling, practitioners may be understandably reluctant to reduce flows to less than these recommendations and deliver sevoflurane “off-label.” In another article on page 57 of this Newsletter, Brian Thomas JD, vice-president for Risk Management, Preferred Physicians Medical, provides some guidance on the actual medicolegal concerns associated with off-label medication administration. This article will briefly review the science that clearly indicates a lower flow limit for sevoflurane is unnecessary.
The major concern for reducing flows when using sevoflurane is the accumulation of Compound A in the circuit and the potential for renal toxicity. While there is no question that sevoflurane can interact with some absorbent formulations to produce Compound A, it has never been shown to result in renal toxicity in humans.6 Furthermore, subsequent to the FDA labelling of sevoflurane, it was clearly shown that Compound A results from the interaction of sevoflurane with absorbents that contain strong bases like potassium hydroxide (KOH) and sodium hydroxide (NaOH).7 It has also been shown that eliminating the KOH and limiting the NaOH to less than 2% yields an effective absorbent that does not produce Compound A.8 In short, while there is no substantiated concern for patient injury from Compound A, there is no risk of Compound A production when using one of the many carbon dioxide absorbents available that limit the strong base to NaOH <2%. Every absorbent has a safety data sheet that is readily available on the internet and indicates the chemical composition of the absorbent (Figure 2). Any fresh gas flow can be used safely when administering sevoflurane subject to the considerations for oxygen concentration noted previously.
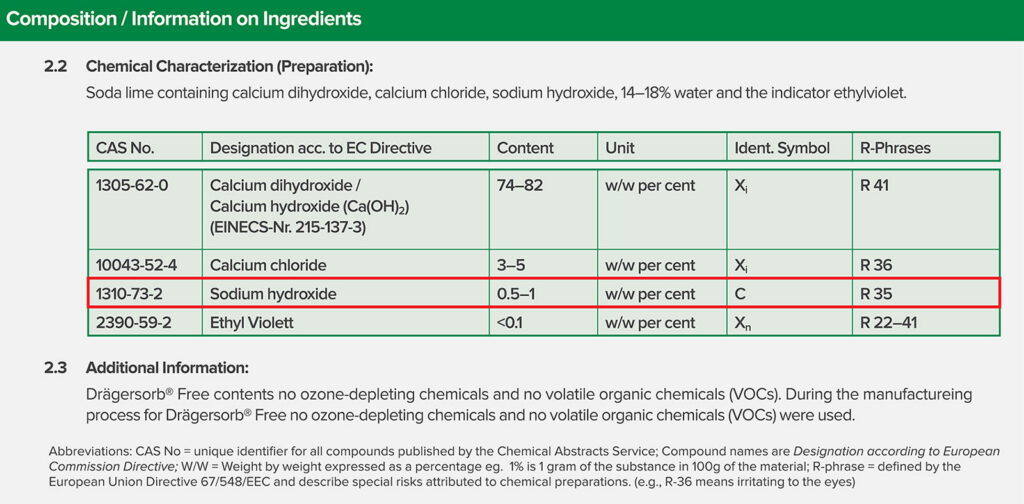
Figure 2: Snapshot of medical safety data sheet for Drägersorb Free. Note that the chemical composition is clearly noted, the sodium hydroxide concentration is 0.5-2%. From https://www.medline.com/media/catalog/Docs/MSDS/MSD_SDSD71242.pdf. Accessed 4/4/2022. Similar safety data sheets can be found in the public domain for any commercially available CO2 absorbent.
Conclusion
The practice of safe and effective low-flow anesthesia is an art that requires the practitioner to understand the capabilities and limitations of the circle system, set fresh gas flow and vaporizer concentrations to estimate patient needs, and continuously monitor the concentrations that result in the circuit. Interested in reducing the waste and pollution in your practice of inhaled anesthetic delivery? Look for the APSF-ASA course on low-flow anesthesia to be available on the APSF website in the fall of 2022.
References
- Ryan SMR, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;11:92–98. 20519425. Accessed April 22, 2022.
- Baum JA. Low flow anaesthesia. 2nd Edition. Butterworth-Heinemann. 2001. pp. 100–105.
- Feldman JM. Managing fresh gas flow to reduce environmental contamination. Anesth Analg. 2012;114:1093–1101. 22415533. Accessed April 22, 2022.
- Waters RM. Carbon dioxide absorption from anaesthetic atmospheres. proceedings of the Royal Society of Medicine. 1936;30:1–12. https://journals.sagepub.com/doi/pdf/10.1177/003591573603000102. Accessed April 22, 2022.
- Ultane (Sevoflurane). Revised 09/01/2003. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020478s016lbl.pdf. Accessed March 13, 2022.
- Sondekoppam RV et. Al. The impact of sevoflurane anesthesia on postoperative renal function: a systematic review and meta-analysis of randomized controlled trials. Can J Anaesth. 2020;67:1595–1623. 32812189. Accessed April 22, 2022.
- Keijzer C, Perez R, DeLange J. Compound A and carbon monoxide production from sevoflurane and seven different types of carbon dioxide absorbent in a patient model. Acta Anaesthesiol Scand. 2007;51:31–37. 17096668. Accessed April 22, 2022.
- Kobayashi S, Bito H, et al. Amsorb Plus And Drägersorb Free, two new-generation carbon dioxide absorbents that produce a low compound a concentration while providing sufficient CO2 absorption capacity in simulated sevoflurane anesthesia. J Anesth. 2004;18:277–281. 15549470. Accessed April 22, 2022.

