The Problem
In 2017, Goudra and Singh wrote: “The growing popularity of deep sedation for UGI [upper gastrointestinal] endoscopy has created immense challenges to anesthesiologists. Gastroenterologists have clearly benefited from deep sedation. It allows performing complicated procedures with relative ease. However, research suggests that management of the airway is anything but routine in this setting, and failure to rescue an airway at an appropriate time has led to disastrous consequences.”1
Case 1
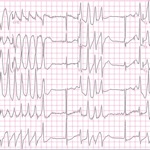
A 57-year-old patient, weighing 91 kg and 183 cm in height, with a medical history significant only for treated hypertension and a remote smoking history, presented for an outpatient UGI endoscopy and a colonoscopy. Vital signs were normal. Monitors and a combined nasal cannula/nasal capnograph at 6 L/min oxygen (O2) were applied and propofol sedation was administered. Shortly after insertion of the esophagoscope, the patient desaturated profoundly to below 50% on pulse oximetry and developed bradycardia and premature ventricular contractions, as well as episodes of ventricular tachycardia.
Code Blue was called. The endoscope was removed, positive pressure ventilation with 100% O2 via an Ambu bag initiated, IV lidocaine administered with ventricular ectopy resolved, and after several minutes of ventilation, O2 saturation reached and remained in the 90s. The esophagoscopy and colonoscopy were canceled. The patient gradually woke up and was transferred to the emergency department for further observation and evaluation. Lab studies including cardiac enzymes were normal, and the patient was discharged home six hours later—a bit tired and sleepy but in stable condition. No formal neuropsychometric testing was performed.
High-Risk Anesthetics
Sedation for upper endoscopies, even “simple” esophagogastroduodenoscopies, should be considered potentially high-risk anesthetics for a number of reasons. First, they are, by definition, “reduced airway access” cases, as the airway is shared by the endoscopist and the anesthesia provider, and because the patient is typically placed in the lateral, semi-prone or prone position, facing away from and greatly reducing the anesthesia provider’s access to the airway. Teeth can be dislodged and the tongue can be pushed into an obstructing position by the bite block or endoscope.
High-Risk Patients
Underlying pathologies—such as esophageal reflux, nausea, vomiting, dysphagia, food impactions, GI bleeding, anemia and obesity/bariatric surgery candidates—place these patients at risk for airway- and respiratory-related complications, as do cardiac pathology in the transesophageal echocardiography (TEE) suite and pulmonary disease in the bronchoscopy suite.
High-Risk Environment and Human Factors
Many upper endoscopies are performed in procedure rooms and endoscopy centers with no access to an anesthesia machine in case of an airway emergency, and are in locations remote from the backup resources available in the OR suite (i.e., non-OR anesthesia, or NORA). Room lights are turned off to allow better visualization of the monitor screens. High case volumes, quick case times and room turnover pressures place time stress on the anesthesia provider, and can lead to the temptation to hurry along the sedation, which can result in under- or oversedation. Computerized anesthesia records can create distractions for the anesthesia provider and can often force them to turn their back to the patient to face the computer screen.
Upper endoscopies often require very deep sedation bordering on general anesthesia to suppress the potent gag, cough and laryngospasm reflexes, especially with initial insertion of the endoscope, and particularly in younger, healthier patients. Subsequently, the level of procedural stimulation—and thus, depth of sedation—can vary suddenly and significantly. Regardless of the specific sedative agent(s) used, deep sedation invariably leads to decreased respiratory drive and relaxation and collapse of the upper airway, particularly in patients with sleep apnea or who are obese, and in small or very ill patients with decreased baseline muscle tone.
Simple Math: Foreign Body Obstruction
It should also be appreciated that upper endoscopies are, by definition, foreign body obstruction cases, as a large foreign body, the endoscope, is placed into the aerodigestive tract, often producing near-total or even total upper airway obstruction.
Some simple math dramatically illustrates the magnitude of this risk. The diameter of commonly used adult esophagogastroscopes is 8.8 to 11 mm. If we apply the formula for the area of a circle, A=pi(r2), it becomes evident that the cross-sectional area of a 9-mm endoscope often exceeds the cross-sectional area of the airway, which is well documented on numerous CT studies,2,3 thereby producing near-total or even total obstruction of the airway in a significant percentage of patients.
Adult TEE probes tend to have even larger diameters than that of esophagogastroscopes. The Guardus Overtube (Steris), a clear plastic tubular device placed over the endoscope by gastroenterologists to create aerodigestive separation for removal of impacted food or foreign bodies, has an even larger outer diameter of 19.5 mm, and is therefore even more likely to produce significant airway obstruction.
Limitations of the O2 Delivery System
One of the biggest challenges in sedation for UGI endoscopy has been the limitation of our supplemental O2 delivery system. The use of traditional O2 face masks for upper endoscopy has been precluded by the fact that the mask impairs access to the mouth for the endoscopist. As a result, the mode of O2 delivery most commonly used is one of our least effective: a nasal cannula, or insufflation via an oral catheter.
Nasal cannulas are recommended to be used at maximal O2 flows of 5 to 6 L/min. Higher rates are not well tolerated because of discomfort and drying of the nares and epistaxis, even over short durations. At O2 flows of 6 L/min, nasal cannulas provide a maximum fraction of inspired O2 (FiO2) of 0.35 to 0.45. In addition, other common clinical conditions, such as hay fever, nasal congestion, nasal polyps, hypertrophied turbinates, septal deviation and mouth breathing, can further reduce the efficacy of the nasal cannula. By contrast, O2 face masks with non-rebreathing reservoirs, at O2 flows of 9 to 15 L/min, comfortably and reliably provide much higher FiO2 levels of 0.90 to 0.95.
As a result of the above challenges and limitations, hypoxia—sometimes profound—during upper endoscopy has perhaps come to be accepted, and even tolerated, as an inherent risk of upper endoscopy under sedation.
A New Paradigm Based on an Old Concept
Because airway management during upper endoscopy under sedation is, by its very nature, high risk, it should be approached much as we approach patients requiring general anesthesia in the OR—by remembering and using the time-tested principles of “safe apneic time” and “maximal preoxygenation.”
Apneic time is defined as the time from when the arterial O2 saturation drops to below 90%, into the steep portion of the hemoglobin–O2 desaturation curve, where it rapidly approaches critically low levels. In healthy adults breathing room air, safe apneic time is less than one minute.4 Morbidly obese patients and patients with decreased capacity for O2 loading (e.g., patients with anemia, pulmonary disease, decreased cardiac output) or increased O2 demand (patients with fever or in a hypermetabolic state) can desaturate much more quickly.5-7
It has been established for decades that the simple technique of maximal preoxygenation can significantly prolong, or even triple, the safe apneic time.5-7 In a classic 1999 editorial in Anesthesiology, Benumof wrote: “The purposes of maximally preoxygenating before the induction of general anesthesia are to provide the maximum time that: 1) a patient can tolerate apnea, and 2) for the professional to solve a cannot-intubate/cannot-oxygenate situation. Moreover, because a cannot-intubate/cannot-oxygenate situation is largely unpredictable, the desirability to maximally preoxygenate is theoretically present for all patients.”7
Benumof vigorously espoused maximal preoxygenation whenever possible. Preoxygenation has become standard practice for many practitioners prior to all general anesthetic inductions.5-7
Maximal preoxygenation is not equivalent to simply reaching 100% saturation on the pulse oximeter; it is a much more thorough O2-loading process. There are several accepted methods of effective maximal preoxygenation.4,7,8 All require high-flow O2 through a well-fitting O2 mask. The originally described technique consisted of five minutes of tidal volume breathing through a high-flow O2 mask. However, this is rather time-consuming and therefore impractical. An equally or more effective and efficient technique is the “8 DB/60 sec” method (eight deep breaths over 60 seconds) described by Baraka et al.7,8
Benumof’s logic and argument for maximum preoxygenation, which has served our patients so well for decades in the potentially high-risk situation of induced apnea in the OR, should be extended to patients undergoing UGI endoscopy under sedation, particularly if this can be done simply and cost-effectively.
A New Device
In recent years, there have been several O2 masks and other devices designed specifically for UGI endoscopy procedures.1 One of these, the Procedural Oxygen Mask (POM Medical), is a clear plastic mask that resembles the traditional familiar clear non-rebreathing O2 mask with an O2 reservoir bag, except that it includes self-sealing oral and nasal endoscopy ports and a capnography sampling port (Figure). The Procedural Oxygen Mask achieves the triple goal of the following: 1) providing reliable FiO2 delivery of 0.90 to 0.95 at O2 flow rates of 10 to 15 L/min when the included O2 reservoir bag is used, 2) allowing easy access to the nose and mouth via the self-sealing endoscopy ports, and 3) providing a continuous capnography sampling port. The mask can be connected to any standard O2 flowmeter.
The high FiO2 levels provided by the Procedural Oxygen Mask allow effective preoxygenation to be achieved efficiently and routinely prior to the start of sedation and endoscopy, and for higher FiO2 levels to be maintained throughout the case compared with nasal cannulas.
Since the incorporation into my practice nine months ago of routine preoxygenation with the Procedural Oxygen Mask for all UGI endoscopies, I have not had a single case of profound or prolonged O2 desaturation. In fact, the new normal using this technique is that O2 saturations remain in the high 90s throughout the procedure. A suggested preoxygenation protocol is described in the Table.
| Table. Protocol for Preoxygenation Prior to Upper Endoscopy | |
| 1. | Apply monitors and position patient. |
| 2. | Insert endoscopy bite block. |
| 3. | Apply Procedural Oxygen Mask using an initial oxygen (O2) flow rate of 15 L/min to fill the O2 reservoir bag, making sure elastic strap is holding mask close to patient’s face to help ensure a good seal, and connect capnography monitoring line. |
| 4. | Ask patient to take 8 deep breaths (DB). O2 saturation should reach and remain at 100% after the first few DB. If not, check the O2 tubing connections and flow rate, gently press mask to patient’s face to achieve a tighter mask seal, and have patient take a few more DB. |
| 5. | Begin sedation, then endoscopy. |
| 6. | If capnography and/or observation of patient indicate profound hypoventilation or apnea, or if O2 saturation drops to 90%, intervene immediately with airway support maneuvers with the endoscope in place, or up to and including removal of the endoscope and bag-valve-mask positive pressure ventilation/oxygenation and advanced airway management, before onset of profound desaturation. |
Case 2
A 58-year-old morbidly obese patient with a body mass index of 43 kg/m2 and on nighttime continuous positive airway pressure (CPAP) mask for severe obstructive sleep apnea, a smoking history of one to 1.5 packs per day, and recent onset of atrial fibrillation presented for outpatient TEE and possible electrical cardioversion. Room air saturation was 93% to 94%. Because of the high risk for respiratory complications, the Procedural Oxygen Mask with O2 reservoir bag was used with an O2 flow rate of 15 L/min, and the patient was fully preoxygenated using the 8 DB/60 sec technique prior to the start of sedation.
Oxygen saturation remained at 98% to 100% throughout the TEE. No mural thrombus was seen on TEE, so the cardiologist elected to proceed with direct current cardioversion. Oxygen saturation remained at 100%. The cardioversion resulted in profound sinus bradycardia, which progressed to asystole. Intravenous atropine was administered and preparations were made to begin chest compressions, but the rhythm returned to sinus tachycardia. The first pulse oximetry reading after return of spontaneous circulation was 97%, which increased to and remained at 100%. The patient woke up almost immediately and was alert and oriented and remained in sinus rhythm, so was admitted to the cardiac unit for overnight rhythm monitoring and discharged to home the next morning.
Conclusion
Capnography and hypervigilance allow rapid diagnosis of severe hypoventilation, even in a dark room with the patient facing away from us. There are now available O2 masks designed for upper endoscopy. Some provide FiO2 delivery of 0.90 to 0.95 and make the goal of effective preoxygenation prior to the start of deep sedation attainable, thus prolonging safe apneic time to allow intervention before the onset of severe hypoxia. Routine use of an endoscopy O2 mask combined with the technique of maximal preoxygenation can help us strive for a goal of zero tolerance of hypoxia during upper endoscopy.
References
- Goudra B, Singh PM. Airway management during upper GI endoscopic procedures: state of the art review. Dig Dis Sci. 2017;62(1):45-53.
- Avrahami E, Solomonovich A, Englender M. Axial CT measurements of the cross-sectional area of the oropharynx in adults with obstructive sleep apnea syndrome. Am J Neuroradiol. 1996;17(6):1107-1111.
- Li HY, Lo YL, Wang CJ, et al. Dynamic drug-induced sleep computed tomography in adults with obstructive sleep apnea. Sci Rep. 2016;6:35849.
- Drummond GB, Park GR. Arterial oxygen saturation before intubation of the trachea. An assessment of techniques. Br J Anaesth. 1984;56(9):987-993.
- Bouroche G, Bourgain JL. Preoxygenation and general anesthesia: a review. Minerva Anestesiol. 2015;81(8):910-920.
- Sirian R, Wills J. Physiology of apnoea and the benefits of preoxygenation. Contin Educ Anaesth Crit Care Pain. 2009;9(4):105-108.
- Benumof JL. Preoxygenation: best method for both efficacy and efficiency. Anesthesiology. 1999;91(3):603-605.
- Baraka AS, Taha SK, Aouad MT, et al. Preoxygenation: comparison of maximal breathing and tidal volume breathing techniques. Anesthesiology. 1999;91(3):612-616.