Anesthesia Patient Safety Foundation
Dear Editor:
HEPA Filters. Do We Really Know Enough?
The global crisis due to COVID-19 has permeated every aspect of our healthcare systems. Concerns about biohazard of (SARS-CoV-2), spread and contact transmission to patients, healthcare personnel, environment and equipment have been widespread, especially with regards to procedures that generate aerosols (AGP’s).1-3 Transmission of the virus is primarily respiratory in nature. SARS-CoV-2 virion is approximately 120 nanometers in diameter (0.06-0.14 μm), and travels from person to person in biological carrier particles such as droplets or aerosols. 2,3 Recommendations with regards to adequate levels of PPE, handwashing, surface cleaning and decontamination, precautions during airway management procedures have been discussed extensively during the pandemic.4-6 As with other respiratory transmissible diseases we rely on two important filtering systems: circuit filters when artificial breathing systems are used in the operating room and/or intensive care units (ICU) and face-mask respirators.
However, things are a bit complicated:
- Anesthesia machines and mechanical ventilators require filters for air quality purification and cross-contamination prevention. The efficiency standard of such filters is termed HEPA for High-efficiency particulate air/ high-efficiency particulate absorbing capacity.7 The ASA recommends placement of HEPA filters between the Y-piece of the breathing circuit and the patient’s mask, endotracheal tube or laryngeal mask airway.8
- European and U.S standards to determine filter efficiency are not the same: European standards use 99.95% removal of particles with a diameter of 0.3 μm in diameter, while the U.S uses 99.97%.9
- Face mask efficiency is determined by the level of particle penetration. An N95 mask for example removes at least 95% of 300 nm particles using an airflow rate of 85 liters/min.10 Face mask respirators are regulated according to U.S National Institute for Occupational Safety and Health (NIOSH) and internationally recognized standards and testing.
- Filters in breathing circuits and anesthesia machines are not regulated. There is no national or international standard test for filters in breathing circuits. Since there is no standard testing, are all manufactures reporting the same when discussing the level of efficiency? 11
- Are current available filters adequate for COVID19?
- Because many COVID-19 patients require prolonged mechanical ventilation, how often should these filters be changed in the ICU?
- What should healthcare professionals do in case of filter shortages?
These are some of the pressing questions with regards to HEPA filters, that I would like to see discussed.
Thank you,
Felipe Urdaneta
Clinical Professor Anesthesiology
University of Florida/NFSGVHS
Gainesville, Florida
*Disclosure: I am a consultant for Medtronic and member of the Advisory Board for Vyaire and have received speaker honoraria on their behalf.
References
- Canova V, Lederer Schlpfer H, Piso RJ, et al. Transmission risk of SARS-CoV-2 to healthcare workers –observational results of a primary care hospital contact tracing. Swiss Medical Weekly. 2020;150:1-5.
- Asadi S, Bouvier N, Wexler AS, Ristenpart WD. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci Technol. 2020;0(0):1-4.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-1567.
- Asenjo JF. Safer intubation and extubation of patients with COVID-19. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 2020:1-3.
- Chia SE, Koh D, Fones C, et al. Appropriate use of personal protective equipment among healthcare workers in public sector hospitals and primary healthcare polyclinics during the SARS outbreak in Singapore. Occup Environ Med. 2005;62:473-477.
- Sorbello M, El-Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75:724-732.
- First MW. Hepa Filters. Journal of the American Biological Safety Association. 1998;3:33-42.
- https://www.asahq.org/about-asa/governance-and-committees/asa-committees/committee-on-occupational-health/coronavirus. COVID-19Information for Health Care Professionals. Published 2020. Accessed June 2020, 2020.
- Wikipedia. HEPA. https://en.wikipedia.org/wiki/HEPA. Accessed June 9-2020, 2020.
- Haghighat F, Bahloul A, Lara J, Mostofi R, Mahdavi A. Development of a Procedure to Measure the Effectiveness of N95 Respirator Filters against Nanoparticles. 2012.
- https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/default.html. NIOSH-Approved Particulate Filtering Facepiece Respirators. Accessed June 9, 2020.
Dear Reader:
Breathing Circuit Filters in the Era of Covid-19
We thank Dr. Urdaneta for his letter, which highlights some of the confusion that has arisen regarding the use of anesthesia breathing system filters in response to the COVID-19 pandemic. The APSF website has a page with FAQ on Anesthesia Machine Use, Protection, and Decontamination During the COVID-19 Pandemic that was first published in March 2020, and has since been updated a number of times, and translated into other languages. While it summarizes the best strategy for protecting the anesthesia machine from contamination by a potentially infected patient, it does not provide some of the details behind the recommendations. These details, such as the risk of patient trans-infection via the breathing system, modes of respiratory virus transmission, physics of filtration, types of filters used in anesthesia, and standardized tests and specifications of filters will be covered here in an effort to answer Dr. Urdaneta’s questions and clear up similar confusions among our readership.
Risk of patient cross-infection via the breathing system
Circle breathing systems present a hypothetical risk of patient cross-infection due to rebreathing of previously exhaled gases. Prior to the 1990s, anesthesia breathing system filters were not routinely used and it was thought that cross-infection of patients was prevented by passage of exhaled gas through the alkaline carbon dioxide absorbent.1 However, breathing circuit filters became increasingly used in the 1990s2 after a report of 9 cases of cross-infection by hepatitis C attributed to contaminated anesthesia breathing systems.3 Review of the literature shows conflicting evidence for the potential for cross-infection; there have been almost no documented cases, but in-vitro tests demonstrate the possibility.4, 5, 6 In any case, breathing system filters are recommended by a number of anesthesia societies when breathing circuits are routinely used for more than one patient. 7, 8
Modes of respiratory virus transmission
COVID-19 (SARS-CoV-2) is transmitted primarily via the respiratory route, as are Severe Acute Respiratory Syndrome (SARS-CoV), Middle-East Respiratory Syndrome-associated coronavirus (MERS-CoV), and other coronaviruses. It is transmitted via droplets, which are more than 20 microns in diameter, and via aerosols which are less than 5-10 microns in diameter.9 Droplets tend to fall due to gravity, whereas aerosol particles float in air and follow airstreams. Intermediate-sized particles share some properties of droplets and aerosols. Droplet nuclei that also follow airstreams are generated by rapid evaporation of small droplets. Droplets, aerosols, and intermediate-sized particles are generated during coughing, sneezing, and talking, whereas primarily aerosols are generated during passive breathing. It is important to realize that respiratory viruses are not transmitted by isolated virus particles floating in air, but by viruses that are contained within larger particles. Droplets and some intermediate-sized particles can settle on surfaces, potentially leading to surface transmission via touching of the eyes and other mucous membranes.
No studies have been performed to estimate the rate that virus particles are exhaled by SARS-CoV-2 infected patients. However, one study that quantified the rate of exhalation of other respiratory virus particles, found that seasonal coronavirus infected patients exhaled and coughed out 0 to 200,000 virus particles per hour.10
While a single viral particle can theoretically result in systemic infection, the chance of infection increases with the duration and magnitude of viral exposure.11
Physics of filtration
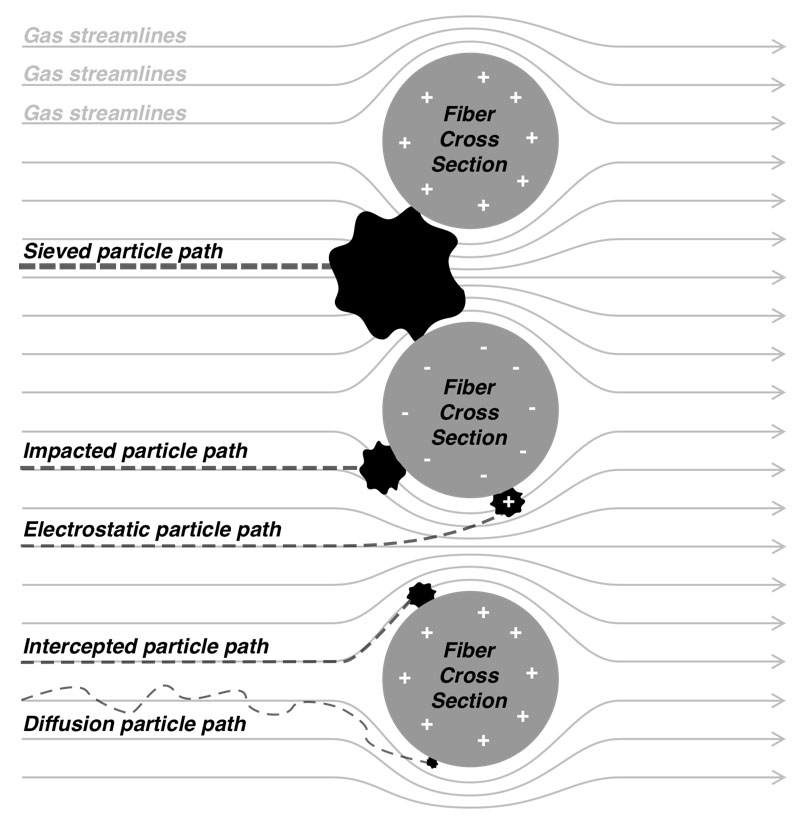
People generally understand the physics of sieve filtration, whereby a particle that is larger than the smallest holes cannot pass through a filter. This is an observable phenomenon, in strainers for example. However, other forces come into play with very small particles (e.g., <2 microns diameter).12 At these small sizes, particles tend to adhere to the filter material once they make contact, even if they could fit through openings in the filter. There are four basic mechanisms whereby particles make contact with the filter material. Particles in the range of 0.1‑1 micron can directly impact filter strands through a process called inertial impaction. Particles in the range of 0.05‑1 micron can tangentially contact filter strands as they pass closely by through a process called interception. As particles get smaller, they increasingly exhibit Brownian motion in addition to moving with air flow – and can contact filter material as a consequence of this erratic movement through a process called diffusion. Finally, any small charged particle can be attracted to the charged surface of filter material through a process called electrostatic attraction. Figure 1 illustrates each of these phenomenon, and Figure 2 shows how the sum of these phenomena affects the overall efficiency of a filter. Note that for most air filters, particles around 0.3 microns (i.e., 300 nanometers) in size are the most difficult to trap – particles that are larger or smaller are easier to catch.
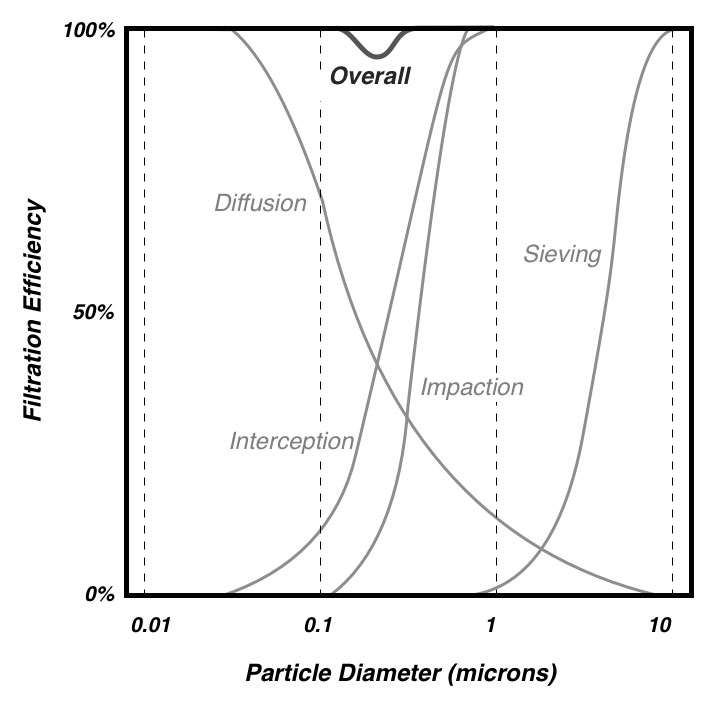
Figure 2: Individual filtration phenomena sum to yield overall filtration for different size particles. Note that the lowest efficiency is around 0.3 microns.
Types of filters used in anesthesia breathing systems
A number of breathing system filter types are available
Pleated mechanical filters
Pleated mechanical filters contain a thick sheet of tightly packed, randomly oriented, bonded hydrophobic fibers that capture particulates within the depth of the filter. The filter material is pleated to increase the surface area and decrease resistance to airflow. They typically have a very high filtration efficiency and may also provide some heat and moisture exchange when placed close to the airway, at a site of two-way airflow. When used in a humid environment, their filtration efficiency and resistance to airflow can get better or worse; they still tend to be highly effective when damp.13 Liquids do not easily pass through pleated mechanical filters.14 Mechanical filters tend to cost more, and have a higher internal volume than electrostatic filters. Mechanical filters are rated by the percent of particles that they trap.
Electrostatic filters
Electrostatic filters contain a thin sheet of electrostatic fibers less tightly woven than pleated filters. Their resistance to airflow is less for a given surface area, so they need not be pleated. Electrostatic filters are so named because they have an electrostatic charge that helps to attract and trap particles. Electrostatic filters typically have 1000-fold lower filtration efficiencies than pleated mechanical filters.13 Their filtration efficiency can get better or worse in a humid environment as can their resistance to airflow. Liquids can easily pass through an electrostatic filter.14 Electrostatic filters are rated by the percent of particles that they trap.
Heat and moisture exchange filters (HMEF)
Heat and moisture exchange (HME) devices can contain an electrostatic or a pleated mechanical filter, in which case they are denoted HMEF. By themselves, HMEs provide no filtering. HMEs and HMEFs are only effective for humidification when placed close to the airway, at a site of two-way airflow, where they absorb water during exhalation and release it during inhalation.15 They are rated either 1) by the absolute humidity of the inhaled gas, or 2) by the moisture loss from a test apparatus representing the lung.
Membrane filters
A completely different type of filter is used in respiratory gas analyzers to prevent fluid entry into the analyzer chamber. While not classified as breathing circuit filters, hydrophobic membrane filters are commonly included in water traps because they allow gas to pass when dry, but become occlusive when wet. Membrane filters are made from liquid polymers such as Polytetrafluoroethylene (PTFE) or Polyester (PETE) that form pores or channels as they solidify – these ultra-small pores and channels also prevent particle passage primarily by sieving. Membrane filters are rated by pore-size.
Standardized tests and specifications of filters
Particle Filtration
There is one single international standard for testing the filtration efficiency of breathing system filters, ISO 23328-1: Breathing system filters for anaesthetic and respiratory use.16 The standard describes a method, the salt test method, that quantifies the number of airborne sodium chloride particles of the most penetrating size range of 0.1 to 0.3 microns that pass through the filter during a short-term challenge at airflow rates likely to be encountered during the intended use. Pediatric and adult filters are challenged with either 0.1 mg or 0.2 mg of sodium chloride particles at 15 L/min or 30 L/min, respectively. Filters are pre-conditioned in humidified air to simulate a period of clinical use before they are tested. Non-electrostatically charged dry salt particles are used for the challenge, because they represent particles that are the most difficult to trap. The method does not assess the filtration performance for droplets and aerosols, nor does it proport to test the filtration performance for microorganisms. It is for comparison purposes only, and has no proven clinical relevance. For this, and other reasons, the standard contains no threshold for minimum performance of breathing system filter efficiency. The test results are expressed as the percent filtration efficiency, which is the percent of particles in the challenge that do not pass through the filter. For example, if the filter is challenged with 10 million (107) particles, and 1000 (103) particles are detected on the other side, then the percent filtration efficiency is 100 * (1 – 103 / 107) = 99.99%.
Entirely different standards are used for testing and rating other types of filters. Notably, the National Institute for Occupational Health and Safety, developed NIOSH 42 CFR Part 84: Respiratory Protective Devices17 as a method to test and rate non-powered air-purifying respirators. Three classes of filters are designated, N-, R-, and P-series, with three levels of filtration efficiency, 95%, 99%, and 99.97%, in each class. N-series respirators that are used in healthcare are challenged with 200 mg of non-electrostatically charged dry sodium chloride particles that are 0.1 to 0.3 microns in size at a flow rate of 85 L/min.18 This is a similar but more challenging test than ISO 23328-1 due to the higher particle mass and flow rate. Another notable filtration testing standard is IEST-RP-CC001: HEPA and ULPA Filters,19 which is used to provide a basis for agreement between customers and suppliers for filters used in clean air devices and clean rooms. Eleven levels of filter performance and six grades of filter construction are described for applications that require extremely high filter efficiency for sub-micrometer particles. HEPA refers to high-efficiency particle air filters that remove 99.97% of particles whose diameter is equal to 0.3 microns. However, it is not appropriate to apply this term to breathing circuit filters because the test methods are different.
Microorganism Filtration
Some breathing system filter product literature contains statements about bacterial and or viral filtration efficiency. There is no standard test for determining the bacterial and or viral filtration efficiency of breathing circuit filters, but there are standard methods for determining this for other types of filters. One of these is ASTM F2101 – 19: Standard test method for evaluating the bacterial filtration efficiency (BFE) of medical face mask materials, using a biological aerosol of Staphylococcus aureus.20 A similar procedure using biologic aerosols of Bacillus subtilis or MS-2 coliphage to test breathing system filters is described by Wilkes et al.21 and is the same as that in Draft BS EN 13328-1 (which never got past the draft stage) . In both procedures, suspensions of bacteria or viruses are aerosolized to a mean liquid particle size of 3.0‑microns and drawn through the filter material by a downstream vacuum. Anything that passes through the filter is captured either into nutrient broth or onto culture plates. Serial dilution of the broth or a system of cascading impactors is used to create increasingly less-concentrated material to be cultured on agar or cell-culture plates, thus allowing assessment of a wide range of colony counts. A percent filtration efficiency is calculated by dividing the number of cultured particles downstream of the filter by the number in the upstream challenge. At face value, these methods might seem more clinically relevant than the salt test method. They use larger sized fluid particles than the salt test method. The fluid particles may be electrostatically charged. Only viable microorganisms are counted. However, these methods are less reproduceable. In general, the same filter will have greater percent filtration efficiencies for bacteria, than for viruses, than for salt particles.
Bubble point testing
Membrane filters are rated by pore size, which is indirectly determined using the bubble point test. The bubble point test is based on the principle that liquid is held in the pores of the filter by surface tension and capillary forces, and that the minimum pressure required to force liquid out of the pores is related to the pore diameter. To perform the test, a filter is held in an airtight casing and submerged in water so that it is wet on both sides. Air at increasing pressure is applied to one side of the filter until a continuous stream of air bubbles escapes from the other side. The pore size is inversely related to the minimum pressure at which the bubbles start escaping, and can be calculated.22 Membrane pore size, as determined by image analysis, correlates well with pore size as determined by the bubble point test.23 However, pore size cannot be used as a surrogate for particle or pathogen filtration efficiency. Hydrophilic 0.22-micron membrane filters are commonly used to sterilize pharmaceuticals, and to maintain sterility of epidural infusions. Some 0.2-micron hydrophobic membrane filters (e.g., those in the GE DFend Pro, Draeger Watertrap 2, and Covidien FilterLine water traps) have been independently tested, and have a viral filtration efficiency of 99.99% or greater.
Clinical recommendations and need for future research
In 2003, the United States Center for Disease Control stated, “No recommendation can be made for placing a bacterial filter in the breathing system or patient circuit of anesthesia equipment”, citing now 40-year old studies that showed failure of sterile breathing circuits or breathing system filters to reduce the incidence of postoperative pneumonia.24 There is no current regulation to use breathing system filters on anesthesia machines. However, it seems prudent to prevent, as best as possible, the cross-infection of patients with SARS-CoV-2 during this COVID-19 pandemic. There are sparse reports of cross-infection from contaminated anesthesia machines prior to SARS, MERS, and COVID-19, but the risk from these pathogens is not currently known. Out of an abundance of caution and informed by existing knowledge, the APSF and ASA recommend using breathing circuit filters, recognizing that the science is incomplete.
We do know that adding breathing system filters is not without risk.25, 26 Depending on placement, they can add dead space which increases carbon dioxide rebreathing and slows inhalation induction and emergence. They increase resistance to inspiratory and/or expiratory flow, which increases spontaneous work of breathing, and affects pulmonary mechanics (testing methods are described in international standard ISO 9360-1)27. Filters can become obstructed leading to life threatening hypoventilation and barotrauma. They add weight to the breathing circuit and add sites for accidental breathing circuit disconnection. Some breathing system filters, especially those with an integrated HME, help to conserve inspired humidity; but the corollary is that they decrease carbon dioxide absorbent humidity, which could theoretically increase the production of toxic metabolites.
The filtration efficiency required to prevent infection from exhaled viruses via the breathing circuit is not known. If a patient exhales 200,000 virus particles per hour, then an electrostatic filter that traps 99.9% of them will allow only 200 to pass. Placing two of these filters in series (e.g., one at the airway and one on the expiratory limb) will multiply the filtration efficiency to 99.9999%, making the risk of virus passage almost nil, but will double the resistance to flow. Using a single higher efficiency (e.g., 99.9999%) pleated mechanical breathing system filter at the airway will capture the same number of viruses and introduce less airway resistance than two electrostatic filters in series, but may increase the dead space. To define the minimum filtration efficiency to prevent cross infection, it would be useful to sample anesthesia machines and ventilators for the presence of virus upstream and downstream of filters after treating SARS-CoV-2 positive patients. The absence of virus downstream would be reassuring. However, even if virus is detected past the filters, it is not clear that it would cause infection.
Clinicians should know the specifications for the breathing system filters available to them. These can be found from the manufacturer’s web site or help line, in product literature, online, and in journal articles.13, 14 Important specifications are:
- bacterial and viral filtration efficiency (% – higher is better),
- NaCl or salt filtration efficiency (% – higher is better),
- resistance to flow (pressure drop in Pa or cmH2O at a given airflow rate in L/min – lower is better),
- how the former specifications are affected by filter conditioning in humidity,
- internal volume (ml – lower is better), and
- humidification
- (moisture loss in mgH2O/L of air – lower is better), or
- (moisture output in mgH2O/L of air – higher is better).
Note that some publications list evaluations that were done 10 or 20 years ago, and that products may change, or be manufactured or distributed by different companies.
Robert G. Loeb, MD
Clinical Professor of Anesthesiology
University of Florida College of Medicine
Disclosure: Masimo Technology Advisory Board
References
- Murphy PM, Fitzgeorge RB, Barrett RF. Viability and distribution of bacteria after passage through a circle anaesthetic system. British journal of anaesthesia. 1991 Mar 1;66:300-4.
- Atkinson MC, Girgis Y, Broome IJ. Extent and practicalities of filter use in anaesthetic breathing circuits and attitudes towards their use: a postal survey of UK hospitals. Anaesthesia. 1999;54:37-41.
- Chant K, Kociuba K, Munro R, Crone S, Kerridge R, Quin J, Wyland M, Miller G, Turner I, Brown J, Baird L. Investigation of possible patient-to-patient transmission of hepatitis C in a hospital. New South Wales Public Health Bulletin. 1994;5:47-51.
- Spertini V, Borsoi L, Berger J, Blacky A, Dieb-Elschahawi M, Assadian O. Bacterial contamination of anesthesia machines’ internal breathing-circuit-systems. GMS Krankenhaushygiene interdisziplinär. 2011;6: 1-5.
- Lloyd G, Howells J, Liddle C, Klineberg PL. Barriers to hepatitis C transmission within breathing systems: efficacy of a pleated hydrophobic filter. Anaesthesia and intensive care. 1997;25:235-8.
- Heinsen A, Bendtsen F, Fomsgaard A. A phylogenetic analysis elucidating a case of patient-to-patient transmission of hepatitis C virus during surgery. Journal of Hospital Infection 2000; 46: 309–13.
- Australian & New Zealand College of Anaesthetists. PS28 Guideline on infection control in anaesthesia 2015. Available at: https://www.anzca.edu.au/safety-advocacy/standards-of-practice/policies,-statements,-and-guidelines (Accessed 7/6/20)
- Association of Anaesthetists of Great Britain & Ireland. Guidelines: Infection prevention and control 2020. https://anaesthetists.org/Portals/0/PDFs/Guidelines%20PDFs/Infection_Control_Guideline_FINAL%202020.pdf?ver=2020-01-20-105932-143 (Accessed 7/6/20)
- Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC infectious diseases. 2019;19:101.
- Leung NH, Chu DK, Shiu EY, Chan KH, McDevitt JJ, Hau BJ, Yen HL, Li Y, Ip DK, Peiris JM, Seto WH. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature medicine. 2020;26:676-80.
- Nicas M, Hubbard AE, Jones RM, Reingold AL. The infectious dose of variola (smallpox) virus. Applied Biosafety. 2004;9:118-27.
- Hakobyan NA. Introduction to basics of submicron aerosol particles filtration theory via ultrafine fiber media. Armenian Journal of Physics. 2015;8:140-51.
- Wilkes A. Breathing System Filters: an assessment of 104 breathing system filters. MHRA Evaluation 04005. March 2004 https://www.psnetwork.org/wp-content/uploads/2018/01/An-assessment-of-104-breathing-system-filters-MHRA-Evaluation-04005-2004-.pdf (Accessed 7/6/20)
- Wilkes AR. The ability of breathing system filters to prevent liquid contamination of breathing systems: a laboratory study* APPARATUS. Anaesthesia. 2002;57:33-9.
- Wilkes AR. Heat and moisture exchangers and breathing system filters: their use in anaesthesia and intensive care. Part 1–history, principles and efficiency. Anaesthesia. 2011 ;66:31-9.
- International Organization for Standardization. Breathing system filters for anaesthetic and respiratory use — Part 1: Salt test method to assess filtration performance. (ISO 23328-1:2003) https://www.iso.org/standard/35330.html (Accessed 7/6/20)
- Department of Health and Human Services. 42 CFR Part 84 Respiratory protective devices; final rules and notice. Federal Register Volume 60, Number 110 (Thursday, June 8, 1995). https://www.govinfo.gov/content/pkg/FR-1995-06-08/html/95-13287.htm (Accessed 7/6/20)
- National Institute for Occupational Safety and Health. Determination of particulate filter efficiency level for N95 series filters against solid particulates for non-powered, air-purifying respirators standard testing procedure (STP). https://www.cdc.gov/niosh/npptl/stps/pdfs/TEB-APR-STP-0059-508.pdf (Accessed 7/6/20)
- Institute for Environmental Sciences and Technology. HEPA and ULPA Filters. (IEST-RP-CC001) https://www.iest.org/Standards-RPs/Recommended-Practices/IEST-RP-CC001 (Accessed 7/6/20)
- ASTM International. Standard test method for evaluating the bacterial filtration efficiency (BFE) of medical face mask materials, using a biological aerosol of Staphylococcus aureus. (F2101 – 19) https://compass.astm.org/EDIT/html_annot.cgi?F2101+19 (Accessed 7/6/20)
- Wilkes AR, Benbough JE, Speight SE, Harmer M. The bacterial and viral filtration performance of breathing system filters. Anaesthesia. 2000 May 29;55:458-65.
- Emory SF. Principles of integrity-testing hydrophilic microporous membrane filters. Part I. Pharm Tech. 1989;13:68–77.
- Hernández AC, Calvo JI, Prádanos P, Tejerina F. Pore size distributions in microporous membranes. A critical analysis of the bubble point extended method. Journal of Membrane Science. 1996;112:1-2.
- CDC Healthcare Infection Control Practices Advisory Committee. Guidelines for Preventing Health-Care–Associated Pneumonia, 2003. MMWR 53(RR03); 1-36, 2004
- Lawes EG. Hidden hazards and dangers associated with the use of HME/filters in breathing circuits. Their effect on toxic metabolite production, pulse oximetry and airway resistance. British journal of anaesthesia. 2003;91:249-64.
- Wilkes AR. Heat and moisture exchangers and breathing system filters: their use in anaesthesia and intensive care. Part 2–practical use, including problems, and their use with paediatric patients. Anaesthesia. 2011;66:40-51.
- International Standards Organization. Anaesthetic and respiratory equipment—heat and moisture exchangers (HMEs) for humidifying respired gases in humans—part 1: HMEs for use with tracheostomized patients having minimal tidal volume of 250 ml (ISO 9360-2: 2001).


Leave a Reply
You must be logged in to post a comment.