In contradiction to the advances in medicine in recent decades, infectious diseases are on the rise.1 Environmental changes due to population shifts, climate change and microbial adaptations have prompted the spread of emerging and reemerging infections.1,2 These diseases can present with airway complications, respiratory and cardiovascular impairment, and unusual neurologic and neuromuscular manifestations, posing challenging scenarios to the anesthesiologist and health care professionals (HCPs) involved in patient care.3
Considering the severity of many highly infectious diseases (e.g., measles, influenza, tuberculosis, severe acute respiratory syndrome, Middle East respiratory syndrome–coronavirus and Ebola), the early identification of symptoms, prompt isolation precautions and other preventive measures are of central importance to containing and mitigating the impact on public health and HCPs.3,4
Infectious Agents: Measles
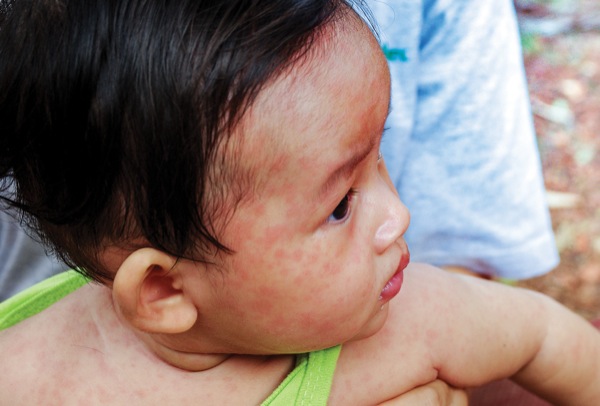
Measles serves as an excellent, albeit unfortunate, example of a highly infectious viral respiratory agent. In the decade before the live measles vaccine was licensed in 1963, an average of 549,000 cases and 495 deaths were reported annually in the United States.5 Almost four decades later, measles was declared eliminated from the country, and it was aggressively fought worldwide (Figure 1).5,6
Unexpectedly, two years ago, measles cases around the world started to re-emerge.6 Although gaps in vaccination due to poverty were the primary cause, rumors, anti-vaccine activists and missteps by some vaccine companies have all favored this rebound.6 The reemergence of the virus occurred in several parts of the world, reaching 63,000 cases in India, 31,000 in Pakistan, 12,000 in Yemen, 10,000 in Brazil, and 5,700 in Venezuela.6 In the European region, despite an increase in vaccination rates, a record number of 82,596 people in 47 of the 53 countries were affected by the virus in 2018.7
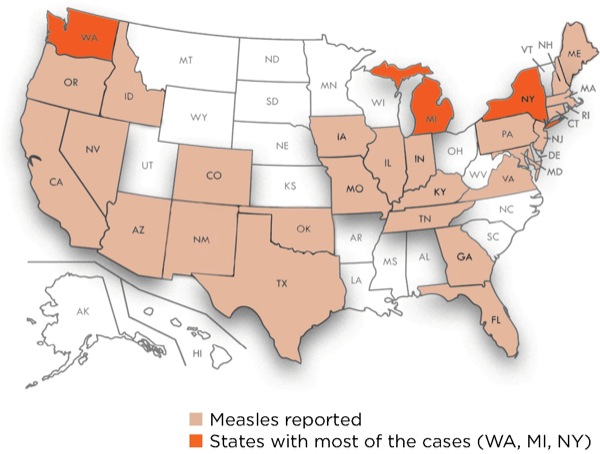
Outbreaks also have occurred in the United States. In 2018, 372 cases from 17 separate outbreaks were reported, and the country is now experiencing the largest outbreak of measles in a quarter century: 1,123 cases in 28 states, as of July 11, 2019 (Figure 2). This is the highest number of cases reported in the United States since 1992, including when the disease was declared eliminated in 2000.8 The majority of the cases have been reported in Michigan, New York and Washington.9
Airway Management
From the anesthesiologist’s perspective, the clinical significance of highly infectious respiratory diseases relates to the potential for early respiratory complications and the increased risk for pathogen transmission.3,10-13 Regarding respiratory symptoms, measles-infected patients can present with dyspnea, severe hypoxemia and significant upper airway obstruction at the time of admission.14-16 Airway impairment can range from mild inspiratory stridor with nasal flaring to frank obstruction and respiratory arrest.15 The need for mechanical ventilation is usually related to respiratory failure due to measles pneumonitis-related complications and measles-induced neurologic complications.16 Pneumonitis, with or without bacterial superinfection, is often the major complication leading to admission to an ICU in measles-infected patients.16
In these severe cases, personal experience and equipment availability should guide the selection of the intubation technique, whether it is direct laryngoscopy, video laryngoscopy or fiber-optic bronchoscopy.3 Measles patients also can present with pharyngitis, as can other infectious agents, such as the diphtheria-causing Corynebacterium diphtheriae.3,16 In such scenarios, the oropharynx inflammation can lead to a friable airway mucosa that can easily become edematous and bleed during airway manipulation. Measles-related neurologic complications can appear early in the disease course and further impair airway management. Nuchal rigidity, bilateral upper limb clonus, seizures, myelitis and encephalitis can appear as soon as four days after measles onset.16
Protective Measures
Close contact with patient airway secretions places HCPs on the front line of epidemics and at greater risk for acquiring respiratory illnesses.10-13,17 Airway management procedures, notably endotracheal intubations, are associated with high rates of dissemination of airborne pathogens due to droplet and aerosol formation.4,18 Aerosols (≤5 mcm) may remain suspended in air for long periods of time and, as a result, also may be transmitted over great distances. This mode of transmission is exemplified by the measles virus, which can remain infectious in the air for up to two hours after an infected person leaves the area.19 In contrast to aerosols, droplets (>5 mcm) are likely to settle quickly on a surface, typically within three feet of the source.18,20,21
To contain the spread of highly infectious diseases, different levels of protective measures are required. In the case of vaccine-preventable diseases, such as measles, the CDC Advisory Committee on Immunization Practices (ACIP) recommends preemployment determination of evidence of immunity, information that should be documented and readily available (ideally in electronic health records) at the workplace.22-24 Presumptive evidence of immunity to measles for HCPs consists of any of the following:
- written documentation of vaccination with two doses of live measles or measles-mumps-rubella (MMR) vaccine administered at least 28 days apart;
- laboratory confirmation of the disease;
- laboratory evidence of immunity; or
- birth before 1957.19
Another important measure for reducing the rates of transmission and containing outbreaks is early diagnosis. Considering that transmission can occur three days before onset of the measles rash, the recognition of measles’ nonspecific preliminary symptoms (cough, coryza and conjunctivitis) is critical but often missed.23,25 As a result, the delay or even failure to implement preventive measures can expose other people to the virus.23
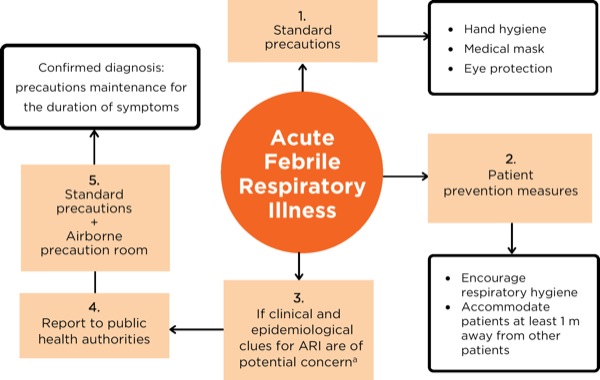
A summarized flow chart of the infection prevention measures for patients with suspected acute febrile respiratory illness is presented in Figure 3. During direct patient contact, HCPs should always adhere to standard exposure precautions and assume that every patient is potentially infected or colonized with an infectious pathogen.26
Standard Precautions
Standard precautions that apply to all respiratory pathogens are hand hygiene, eye protection, the use of gloves and gown, and a respiratory protective device.26 Hand hygiene should be performed before and after all contact with patients or potentially infectious material, and before and after the use of personal protective equipment.26 Alcohol-based rubs or soap and water can be used for hand hygiene; however, the latter is preferred if hands are visibly soiled.26 Ethanol at 95% has been shown to be effective at eliminating the majority of clinically relevant viruses, although the addition of an acidic agent can improve the viricidal activity of alcohol even at lower concentrations.27
Eye protection, such as a face shield or goggles, should be used during procedures that are likely to generate splashes or sprays of blood, secretions or excretions.4 Ocular membranes are both a portal of entry for infections and a potential site for viral replication, even for viruses that do not demonstrate ocular tropism.28
Gloves are indicated when contact with any material that is potentially infected might occur, and the use of double gloves should be considered.26,29 When using double gloves, the outer gloves should be removed immediately after airway manipulation and, as soon as possible, providers should remove the inner gloves and perform hand hygiene.29 Gown use is indicated when there is possible contact with blood, body fluids, secretions or excretions.26
In addition, respiratory protective devices should be used during interactions with patients suspected of being infectious, even though many HCPs often do not follow recommended practices, for numerous reasons.22 The most commonly used respiratory devices designed to prevent the transmission of airborne infectious agents are medical masks and respirators.30Although standard medical masks are designed to obstruct droplets from coming in contact with the wearer, they are not fitted or engineered to perform filtration of smaller airborne infectious agents. These smaller particles, often found in aerosols, can be removed by higher efficiency respirators, which can work as an air-purifying device (filtering the particles) or an atmosphere supplier (supplying clean air). Respirators can be further divided according to their efficiency in removing particles—95%, 99% and 100%—and oil resistance (N=no oil resistance, R=oil resistance, P=oil resistant). Although the use of the 95% efficient respirator or an N95 mask is controversial for several reasons, it is still recommended during most aerosol-generating procedures, especially when a highly infectious agent may be encountered (Figure 4).31-33
In the case of very high-risk procedures, such as endotracheal intubation and extubation, the use of more aggressive protective measures is encouraged. This is especially true in a high-risk setting, such as when treating patients who have infections associated with a high rate of mortality.34 Powered air purifying respirators (PAPRs) provide a higher level of protection than N95 masks without some of the difficulties, such as the need to be fitted.35-37 PAPRs, however, present their own challenges: They are expensive, require proper cleaning and maintenance (not disposable), and can interfere with tasks and intubations.33-36 However, in the context of a highly infectious agent and the concomitant need for a greater level of protection, PAPRs are the equipment of choice.35
Equipment Cleaning and Disinfection
Medical equipment and environmental surfaces can become contaminated with airborne pathogens, and meticulous cleaning and disinfection are mandatory components of an effective infection prevention program.38-40 Cleaning, the removal of foreign material from objects, is normally accomplished with the use of water and detergents and should be performed before disinfection.39 Disinfection should be conducted with an Environmental Protection Agency (EPA)-registered low- or intermediate-level disinfectant.39 Although fundamental, these steps are often suboptimal due to varying levels of training and education, lack of antimicrobial activity against a particular agent, or failure to follow the manufacturer’s recommendations (e.g., contact time with the product).40 By law, users must adopt all applicable label instructions for EPA-registered products.40
The inadequate reprocessing of laryngoscopes also has been associated with infectious disease outbreaks.41 The Society for Healthcare Epidemiology of America recommends that standard reusable laryngoscopes undergo high-level disinfection or sterilization prior to use or be replaced with single-use laryngoscopes.29 Single-use disposable laryngoscopes have evolved in recent years, and their performance and cost may be comparable to reusable laryngoscopes if the costs of high-level decontamination of the latter are considered.29
Besides traditional medical equipment, the widespread use of mobile phones by HCPs has raised concerns over their relationship to nosocomial infections, especially in operating rooms where standards for hygiene are higher.42 Recent studies have reported a prevalence of contaminated mobile phones ranging from 62% to 99%, whereas potential clinical pathogens were detected on up to 75% of phones.42 Alarmingly, routine cleaning of phones was reported by only one-third of HCPs, and just 21% reported using alcohol-containing wipes on their phones.43
Post-Exposure Prophylaxis
In the event of a measles outbreak within a hospital or in the areas served by it or other medical facilities, all HCPs should receive two doses of MMR vaccine unless they have proof of immunity or any contraindication to vaccination.24 Contraindications for receiving the MMR vaccine include a history of anaphylactic reactions to neomycin, history of severe allergic reaction to any component of the vaccine, pregnancy and immunosuppression.19 HCPs who have recently been vaccinated (prior to the outbreak) can continue to work without restrictions. If only one vaccine dose is documented, the HCP can remain at work but should receive the second dose at the appropriate interval (at least 28 days after the first dose).24
In the case of an HCP having direct exposure to a patient with measles, those who cannot readily check or verify their immunity status should be offered post-exposure prophylaxis. The MMR vaccine should be administered in the first 72 hours, or immunoglobulin should be given within six days.
The incubation period of measles averages 10 to 12 days from first exposure to prodrome, and 14 days from exposure to rash (range, seven to 18 days), and its transmissibility begins with the prodrome until three to four days after rash onset.19 Due to these characteristics, personnel without evidence of immunity should be excluded from duty from day 5 after first exposure until day 21 after last exposure, irrespective of the post-exposure vaccine.5 HCPs who exhibit measles symptoms should be restricted from all patient contact and excluded from work for at least four days after the beginning of the rash.24
All HCPs caring for a patient with suspected or confirmed measles should use fit-tested respiratory protection consistent with more aggressive airborne infection control precautions (N95 or higher-level respirators) regardless of any HCP’s presumed immunity status. This is advised because, although rare, the transmission of measles has occurred in an HCP who had serologic evidence of immunity or previously had two doses of vaccine.23 Patients with suspected or confirmed measles preferably should be located in an airborne infection isolation room (AIIR).5 An AIIR is a single-patient room with specific air-handling measures, as recommended by the American Institute of Architects/Facility Guidelines Institute standards, such as:
- negative pressure;
- 12 air exchanges per hour for new construction and six air exchanges per hour for existing facilities; or
- air exhaust sent directly to the outside or recirculated through high-efficiency particulate air filtration before return.
If an AIIR is not available, a single room with a closed door situated away from susceptible contacts may be used.44 If HCPs with documented immunity are available, they are the preferred care providers over susceptible HCPs, who should avoid entering the patient’s room.44
Treatment
There is no specific antiviral therapy for measles. Supportive care can help relieve the symptoms and possible complications, such as bacterial infections. Since vitamin A deficiency contributes to delayed recovery and a higher rate of post-measles complications, vitamin A can be given to children immediately on diagnosis and repeated the next day in severe cases.5,45 Ribavirin has been described as the only antiviral treatment used experimentally in the clinical setting of measles infection after an in vitro study revealed its potential to interfere with the viral replication cycle.16 However, clinical studies of its application in this scenario remain limited.16
Conclusion
Although the trend is unexpected, infectious diseases are on the rise. Measles is a reemerging disease that poses challenges to all HCPs because of its ability to cause severe respiratory complications and its high transmissibility. A multifaceted protective approach that includes vaccination, strong surveillance, proper patient placement, effective environmental cleaning, and appropriate use of protective equipment is vital to mitigate potential threats to the public and the health of HCPs.
References
- Ellwanger JH, Kaminski VL, Chies JAB. Emerging infectious disease prevention: Where should we invest our resources and efforts? J Infect Public Health. 2019;12(3):313-316.
- Millán R, Thomas-Paulose D, Egan DJ. Recognizing and managing emerging infectious diseases in the emergency department. Emerg Med Pract. 2018;20(5):1-20.
- Porteous GH, Hanson NA, Sueda LA, et al. Resurgence of vaccine-preventable diseases in the United States: anesthetic and critical care implications. Anesth Analg. 2016;122(5):1450-1473.
- World Health Organization. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. World Health Organization Guidelines. https://apps.who.int/?iris/?bitstream/?handle/?10665/?112656/?9789241507134_eng.pdf;jsessionid=F43712FE2B146F3A8ED6D1DA1C8DE583?sequence=1. Accessed April 5, 2019.
- CDC. Measles (Rubeola). For Healthcare Professionals. 2018. www.cdc.gov/?measles/?hcp/?index.html. Accessed April 9, 2019.
- McNeil DG. Scientists thought they had measles cornered. They were wrong. The New York Times. April 3, 2019. www.nytimes.com/?2019/?04/?03/?health/?measles-outbreaks-ukraine-israel.html. Accessed April 9, 2019.
- World Health Organization Regional Office for Europe. Measles in Europe: record number of both sick and immunized. February 7, 2019. www.euro.who.int/?en/?media-centre/?sections/?press-releases/?2019/?measles-in-europe-record-number-of-both-sick-and-immunized. Accessed May 2, 2019.
- CDC. Measles (Rubeola). Measles Cases and Outbreaks. 2019. www.cdc.gov/?measles/?cases-outbreaks.html. Accessed July 11, 2019.
- Cai W, Lu D, Reinhard S. Where every measles case has been reported this year. The New York Times – Health. 2019. www.nytimes.com/?interactive/?2019/?04/?30/?health/?measles-outbreak-by-state-map.html?smid=tw-nythealth&smtyp=cur. Accessed May 2, 2019.
- Branch-Elliman W, Savor Price C, McGeer A, et al. Protecting the frontline: designing an infection prevention platform for preventing emerging respiratory viral illnesses in healthcare personnel. Infect Control Hosp Epidemiol. 2015;36(3):336-345.
- Koh D. Occupational health aspects of emerging infections – SARS outbreak affecting healthcare workers. Occup Environ Med. 2018;75(suppl 2):A14. Abstract 9.
- Koh DSQ. Occupational health aspects of emerging infections – the experience from developing countries. Occup Environ Med. 2018;75(suppl 2):A205. Abstract 1599.
- Suwantarat N, Apisarnthanarak A. Risks to healthcare workers with emerging diseases: lessons from MERS-CoV, Ebola, SARS, and avian flu. Curr Opin Infect Dis. 2015;28(4):349-361.
- Abramson O, Dagan R, Tal A, et al. Severe complications of measles requiring intensive care in infants and young children. Arch Pediatr Adolesc Med. 1995;149(11):1237-1240.
- Manning SC, Ridenour B, Brown OE, et al. Measles: an epidemic of upper airway obstruction. Otolaryngol Head Neck Surg. 1991;105(3):415-418.
- Rafat C, Klouche K, Ricard JD, et al. Severe measles infection: the spectrum of disease in 36 critically ill adult patients. Medicine (Baltimore). 2013;92(5):257-272.
- Sandoval C, Barrera A, Ferres M, et al. Infection in health personnel with high and low levels of exposure in a hospital setting during the H1N1 2009 influenza A pandemic. PLoS One. 2016;11(1):e0147271.
- Fernstrom A, Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. J Pathog. 2013;2013:493960.
- CDC. Epidemiology and Prevention of Vaccine-Preventable Diseases. Measles. 2018. www.cdc.gov/?vaccines/?pubs/?pinkbook/?meas.html. Accessed April 21, 2019.
- Bunyan D, Ritchie L, Jenkins D, et al. Respiratory and facial protection: a critical review of recent literature. J Hosp Infect. 2013;85(3):165-169.
- Herfst S, Böhringer M, Karo B, et al. Drivers of airborne human-to-human pathogen transmission. Curr Opin Virol. 2017;22:22-29.
- Krah J, Novak D, Stradtman L. Workplace solutions. Preparedness through daily practice: the myths of respiratory protection in healthcare. 2015. www.cdc.gov/?niosh/?docs/?wp-solutions/?2016-109/?pdfs/?2016-109.pdf?id=10.26616/?NIOSHPUB2016109. Accessed April 21, 2019.
- Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618.
- CDC. Manual for the Surveillance of Vaccine-Preventable Diseases. Chapter 7: Measles. www.cdc.gov/?vaccines/?pubs/?surv-manual/?chpt07-measles.html. Accessed May 2, 2019.
- Porretta A, Quattrone F, Aquino F, et al. A nosocomial measles outbreak in Italy, February-April 2017. Euro Surveill. 2017;22(33):30597.
- CDC. Influenza (Flu). Prevention Strategies for Seasonal Influenza in Healthcare Settings. 2018. www.cdc.gov/?flu/?professionals/?infectioncontrol/?healthcaresettings.htm. Accessed April 20, 2019.
- Kampf G. Efficacy of ethanol against viruses in hand disinfection. J Hosp Infect. 2018;98(4):331-338.
- Belser JA, Gustin KM, Katz JM, et al. Influenza virus infectivity and virulence following ocular-only aerosol inoculation of ferrets. J Virol. 2014;88(17):9647-9654.
- Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area. Infect Control Hosp Epidemiol. 2019;40(1):1-17.
- Coia JE, Ritchie L, Adisesh A, et al. Guidance on the use of respiratory and facial protection equipment. J Hosp Infect. 2013;85(3):170-182.
- Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302(17):1865-1871.
- MacIntyre CR, Wang Q, Cauchemez S, et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses. 2011;5(3):170-179.
- MacIntyre CR, Wang Q, Seale H, et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med. 2013;187(9):960-966.
- CDC. Interim Guidance on Infection Control Measures for 2009 H1N1 Influenza in Healthcare Settings, Including Protection of Healthcare Personnel. 2010. www.cdc.gov/?h1n1flu/?guidelines_infection_control.htm. Accessed April 21, 2019.
- Tompkins BM, Kerchberger JP. Special article: personal protective equipment for care of pandemic influenza patients: a training workshop for the powered air purifying respirator. Anesth Analg. 2010;111(4):933-945.
- MacIntyre CR, Wang Q, Rahman B, et al. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers. Prev Med. 2014;62:1-7.
- Candiotti KA, Rodriguez Y, Shekhter I, et al. A comparison of different types of hazardous material respirators available to anesthesiologists. Am J Disaster Med. 2012;7(4):313-319.
- Rutala WA, Weber DJ. Monitoring and improving the effectiveness of surface cleaning and disinfection. Am J Infect Control. 2016;44(5 suppl):e69-e76.
- Rutala WA, Weber DJ. Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. www.cdc.gov/?infectioncontrol/?pdf/?guidelines/?disinfection-guidelines.pdf. Accessed April 21, 2019.
- Boyce JM. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control. 2016;5:10.
- Muscarella LF. Reassessment of the risk of healthcare-acquired infection during rigid laryngoscopy. J Hosp Infect. 2008;68(2):101-107.
- Chang CH, Chen SY, Lu JJ, et al. Nasal colonization and bacterial contamination of mobile phones carried by medical staff in the operating room. PLoS One. 2017;12(5):e0175811.
- Chao Foong Y, Green M, Zargari A, et al. Mobile phones as a potential vehicle of infection in a hospital setting. J Occup Environ Hyg. 2015;12(10):D232-D235.
- Siegel JD, Rhinehart E, Jackson M, et al, and the Healthcare Infection Control Practices Advisory Committee. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (2007). www.cdc.gov/?infectioncontrol/?guidelines/?isolation/?index.html. Accessed May 6, 2019.
- World Health Organization. Weekly epidemiological record: Measles vaccines: WHO position paper. 2009;84:349-360. www.who.int/?wer/?2009/?wer8435.pdf#page=3. Accessed May 6, 2019.




Leave a Reply
You must be logged in to post a comment.