SUMMARY:Quantitative neuromuscular monitoring is the only reliable means of confirming adequate recovery from neuromuscular blockade and avoiding postoperative residual weakness. Anesthesiologists must familiarize themselves with new monitoring technologies in an effort to reduce complications related suboptimal neuromuscular blockade management.
The Call for Monitoring
When patients become hypotensive in the operating room, the anesthesia professional immediately delivers the necessary treatment. Whether it be intravenous fluids or vasoactive medications, clinicians have been trained to acutely intervene and avoid clinical deterioration. How do anesthesia professionals know that their intervention has been successful? Do they assume that the bolus of phenylephrine was adequate because they are familiar with the pharmacodynamics of this drug and expect all patients to respond in a predictable manner? Do they palpate the carotid artery following a bolus of intravenous fluids to ensure that they have restored intravascular volume and achieved hemodynamic stability? Of course not. In fact, anesthesia professionals go to great lengths to ensure they have accurate devices such as an appropriately sized blood pressure cuff or even an intra-arterial catheter that provides real-time, quantitative blood pressure measurements. This group of providers expects their intervention will have the desired effect, but innate vigilance compels them to verify and not rely on predictive pharmacodynamics or subjective assessments such as palpating the pulse.
This practice pattern must be broadened to neuromuscular blockade management. Administering a neuromuscular blockade antagonist such as sugammadex or neostigmine, waiting several minutes, and then extubating a patient’s trachea without confirming adequate recovery is analogous to administering phenylephrine without rechecking the blood pressure and confirming this intervention was successful. Similarly, palpating the response of the thumb to a train-of-four stimulation with a peripheral nerve stimulator (PNS) and subjectively determining whether adequate recovery has been achieved is a comparable practice to palpating the carotid artery during volume administration. Anesthesia professionals rely on state of the art technologies to maintain homeostasis of patients and must not exclude neuromuscular blockade management from such efforts.
The reluctance among the anesthesia community to adopt quantitative (or objective) monitoring is a curious phenomenon that has sparked its own collection of literature. An international survey of >2500 anesthesiologists revealed significant gaps in knowledge regarding fundamentals of neuromuscular blockade management as respondents answered only 57% of the questions correctly. Of greater concern may be the fact that 92% of respondents that provided incorrect responses were inappropriately confident in their wrong answer.1 There is also an emerging belief that the introduction of sugammadex negates the need for quantitative monitoring. While this reversal agent has certainly allowed for faster neuromuscular blockade antagonism and at deeper levels of blockade, sugammadex administration without monitoring can still result in up to 9.4% of patients having residual weakness at the time of extubation.2 Such knowledge gaps and misplaced confidence have certainly been obstacles, while inconsistent training has also been described as a barrier to monitoring.3 Finally, there has historically been a paucity of user-friendly, reliable quantitative neuromuscular monitors that interested clinicians can access.4
The lack of routine quantitative monitoring is a problem that persists worldwide, but momentum continues to build with renewed interest in this topic among anesthesia professionals. Expert panels have called for routine monitoring,5 while anesthesia societies have established guidelines recommending the use of quantitative monitoring whenever neuromuscular blocking agents (NMBA) are administered.6-8 Industry has responded with new monitors and innovations that should enhance patient safety. This article will review some of the state of the art technologies that are currently available for clinicians seeking to utilize quantitative neuromuscular monitoring.
Monitoring Modalities
The use of a peripheral nerve stimulator (PNS) is qualitative and even experienced anesthesia professionals are unable to reliably detect fade when the train-of-four ratio (TOFR) exceeds 0.4.9 Furthermore, evidenced-based protocols that incorporate targeted NMBA administration, routine neuromuscular blockade antagonism, and the “optimal use” of a peripheral nerve stimulator can still leave 35% of patients with residual weakness.10 The limited role of the PNS should be relegated to a device that is used if there is no access to quantitative monitors or as a device that provides qualitative information while anesthesia professionals transition to quantitative monitors.5
Quantitative monitors are typically classified based on the methods by which the device obtains objective measurements (also known as their monitoring modality). However, such devices can also be classified based on whether they are hand-held, standalone monitors or if they are incorporated into the anesthesia work station. Handheld monitors offer the flexibility to obtain objective measurements outside of the operating room. Postoperative residual weakness is certainly not an intraoperative-specific patient safety threat and portable monitors allow for diagnosis in the recovery room or intensive care unit. Monitors incorporated into the anesthesia workstation consist of integrated modules that allow for seamless communication of objective measurements into the electronic medical record. Understanding the needs of your practice will prove invaluable when choosing a monitoring modality and whether you need a portable or integrated monitor.
Mechanomyography
While not commercially available, every new device is compared to mechanomyography (MMG). This historic gold standard has a cumbersome setup that takes careful calibration as it obtains objective measurements by measuring the isometric contraction force following neurostimulation. When interpreting peer-reviewed literature on new quantitative monitoring technologies, the highest level of evidence currently results from direct comparison with MMG.
Acceleromyography
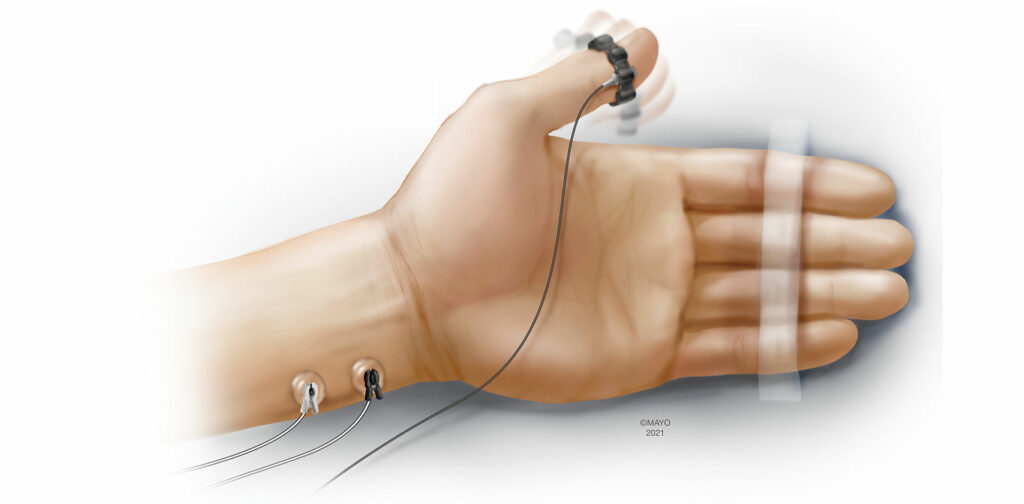
Acceleromyography (AMG) is one of the most investigated and utilized forms of quantitative monitoring.4 Based on Newton’s second law of motion (Force = Mass × Acceleration), AMG objectively measures the response to neurostimulation using a transducer that is fixed to the muscle of interest. Traditionally, standard electrocardiogram (ECG) electrodes are placed over the ulnar nerve and the acceleration of the adductor pollicis muscle is measured following neurostimulation (Figure 1). This configuration is very similar to employing a peripheral nerve stimulator at the hand, except for the additional transducer fixed to the thumb. AMG has also been used at alternative monitoring sites such as the foot (flexor hallucis brevis), and the face (orbicularis oculi/corrugator supercilii). While the setup of AMG can be intuitive, there are important caveats to using this monitoring modality. The “reverse fade” phenomenon in which baseline, unparalyzed TOF exceed 1.0 has been well described when monitoring with AMG.11 While the exact mechanisms remain unclear, reverse fade can have significant implications when determining whether a patient has achieved adequate neuromuscular recovery prior to extubating the trachea. Normalization is a process that places all TOFRs in the context of the baseline TOFR (current TOFR / baseline TOFR) and can account for baseline TOFRs exceeding 1.0. Rather than defining adequate recovery of neuromuscular function as a TOFR >0.9, adequate recovery is truly achieved when the normalized TOFR > 0.9, when measured with AMG.
Furthermore, normalization decreases bias in relation to the MMG. The use of a preload device, which stabilizes the motion of the thumb, and the performance of calibration prior to administration of NMBA, which can also enhance precision of AMG monitoring, are both mandatory when conducting research in this field.12 However, these additional steps are not necessarily required during the course of clinical care. In contrast, routine normalization is strongly encouraged to avoid overestimating the degree of neuromuscular recovery at the end of the operation.
Perhaps the most important caveat to consider when monitoring with AMG is the fact that the muscle of interest (typically the thumb) must be able to freely move following neurostimulation. Tucking the arms during surgical positioning can have a significant impact on the clinician’s ability to obtain reliable measurements with AMG. Additionally, AMG monitoring in awake patients can prove challenging as spontaneous movement at the monitored site can produce artifact.
While there are important nuances clinicians must be familiar with prior to implementing AMG monitoring, recent advances in the modality have made AMG more accessible. Three dimensional transducers are now incorporated into newer AMG devices that allow for better quantification of the complex motion of the thumb following neurostimulation. Also, the incorporation of preload devices into newer devices improves precision without having to obtain and place extra equipment.13 Additionally, wireless configurations of AMG monitors have been developed that utilize bluetooth technology to transmit quantitative measurements from the monitoring site to a display incorporated into the anesthesia workstation (personal communication). AMG monitors are available either as handheld units or modules that can be incorporated into the anesthesia workstation.
Kinemyography
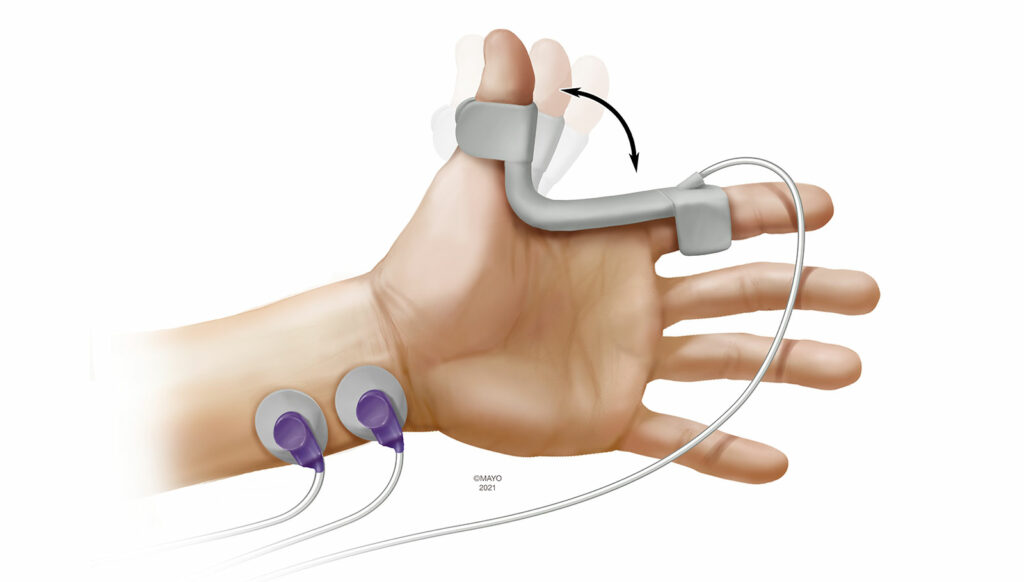
Kinemyography (KMG) is closely related to AMG as a monitoring modality. During KMG monitoring, a piezeoelectric sensor is placed in the groove between the thumb and index (Figure 2). Following ulnar nerve stimulation, the adductor pollicis muscle contracts and the piezoelectric sensor bends. The degree of bend is then translated to objective measurements. The sensor serves as its own preload device and KMG is not subject to the reverse fade phenomenon as with AMG. Previous reports have demonstrated wide limits of agreement between MMG and KMG.14 Like AMG, KMG is also dependent on the thumb being able to freely move and tightly tucking the arms during surgical positioning can preclude its use. Patient movement during emergence can also impact KMG monitoring, as does repositioning the sensor over the course of the perioperative period. Currently, the only available KMG-based device is a module that is incorporated into the anesthesia workstation.
Electromyography
Electromyography (EMG) has been considered by many experts as the new gold standard, given its high level of agreement with MMG15-17 and the fact that EMG provides reliable quantitative measurements even when the arms are restricted during surgical positioning. EMG measures combined muscle action potentials (CMAPs) across the neuromuscular unit rather than motion or any surrogate for motion. The amplitude of CMAPs is directly proportional to the number of activated muscle fibers (and thus the force of contraction). EMG is subject to interference from electrocautery and amplitude of CMAPs can increase 2–3% for every 1°C decrease in skin temperature.18
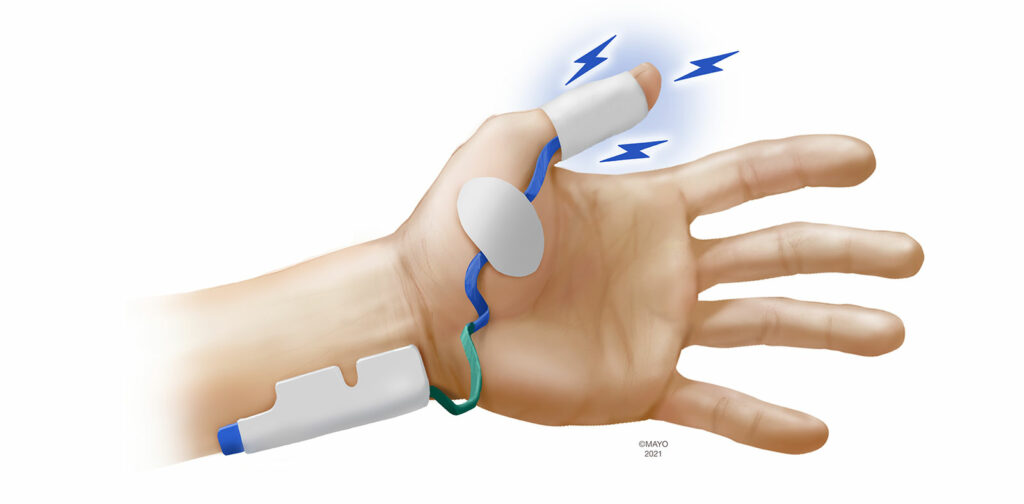
EMG devices are available in either portable, handheld units or incorporated into the anesthesia workstation. Most manufacturers utilize proprietary electrodes to stimulate and measure CMAPs that are placed over the hand. As EMG monitoring is not disrupted when the arms are tucked, seeking alternative sites is not as important with EMG, although monitoring at the foot has been described and is an option should neither hand be available.19 When monitoring at the hand, three muscle groups have been utilized to measure CMAPs following ulnar nerve stimulation. Similar to AMG and KMG, the sensing electrodes can be placed over the adductor pollicis muscle (Figure 3). The first dorsal interosseous muscle, located in the interspace between the thumb and index finger can also be monitored. Finally, the adductor digiti minimi (5th digit) is innervated by the ulnar nerve and is a suitable monitoring site with EMG. Despite being the oldest monitoring modality, there has been significant recent interest in EMG as evidenced by several new EMG-based monitors being introduced to market.
Cuff-based Monitoring
A new device that incorporates objective monitoring within the blood pressure cuff has recently been developed.20 Also referred to as the modified-cuff technique, cuff-based monitoring appears to be inspired by compressomyography, a now-defunct monitoring modality that showed initial promise.21 Cuff-based monitoring involves inflation of the blood pressure cuff to roughly 60 mmHg followed by electrodes within the cuff providing neurostimulation. Pressure changes are detected following muscle contraction and these pressure changes are used to provide clinicians objective data regarding the level of neuromuscular blockade. Early investigations have shown that monitoring the upper arm may have different neuromuscular properties than the distal muscles of the hand and cuff-based monitoring may not be interchangeable with EMG- or AMG-based monitoring at the hand.22 While cuff-based monitoring technology is appealing as it monitors two important parameters (blood pressure and level of neuromuscular blockade), further investigation is warranted to delineate its repeatability and reproducibility across various clinical scenarios.
Implementing Monitoring Into Your Practice
Obstacles certainly exist when implementing significant practice changes, particularly when many in the anesthesia community fail to acknowledge the persistent problem that is postoperative residual weakness. The decision to change and introduce monitoring to your practice can be intimidating as it involves stepping out of one’s comfort zone, putting in extra time, and learning a new skill. Concerns may arise regarding such change negatively impacting workflow and efficiency. Fortunately, the application of quantitative monitors has been demonstrated to only add an additional 19 seconds to the start of a case.23 Once the decision has been made to implement monitoring, the decision on how to proceed can also seem daunting. Certainly, understanding the culture of your practice is critical as described by Todd et al. when this group implemented department-wide EMG monitoring after observing an unacceptable number of patients encountering respiratory distress in the recovery room.24
Becoming familiar with emerging monitoring technologies will certainly improve the chances for successful implementation and changing practice. The specific monitor or modality is just one part of the change as the decision to change practice is much more important and often times is far more challenging. This change will undoubtedly require additional work; however, we owe it to our patients to deliver state of the art care.
References
- Naguib M, Brull SJ, Hunter JM, et al. Anesthesiologists’ overconfidence in their perceived knowledge of neuromuscular monitoring and its relevance to all aspects of medical practice: an international survey. Anesth Analg. 2019;128:1118–1126.
- Kotake Y, Ochiai R, Suzuki T, et al. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg. 2013;117:345–351.
- Thomsen JLD, Marty AP, Wakatsuki S, et al. Barriers and aids to routine neuromuscular monitoring and consistent reversal practice—a qualitative study. Acta Anaesthesiol Scand. 2020;64:1089–1099.
- Soderstrom CM, Eskildsen KZ, Gatke MR, Staehr-Rye AK. Objective neuromuscular monitoring of neuromuscular blockade in Denmark: an online-based survey of current practice. Acta Anaesthesiol Scand. 2017;61:619–626.
- Naguib M, Brull SJ, Kopman AF, et al. Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg. 2018;127:71–80.
- Lucas DN, Russell R, Bamber JH, Elton CD. Recommendations for standards of monitoring during anaesthesia and recovery 2021. Anaesthesia. 2021 Jun 18. doi: 10.1111/anae.15528.
- Dobson G, Chow L, Filteau L, et al. Guidelines to the practice of anesthesia—revised edition 2020. Can J Anaesth. 2019;64:75–91.
- Indications of neuromuscular blockade in anaesthesia. Short text. Ann Fr Anesth Reanim. 2000;19 2:352s–355s.
- Viby-Mogensen J, Jensen NH, Engbaek J, et al. Tactile and visual evaluation of the response to train-of-four nerve stimulation. Anesthesiology. 1985;63:440–443.
- Thilen SR, Ng IC, Cain KC, Treggiari MM, Bhananker SM. Management of rocuronium neuromuscular block using a protocol for qualitative monitoring and reversal with neostigmine. Br J Anaesth. 2018;121:367–377.
- Claudius C, Skovgaard LT, Viby-Mogensen J. Is the performance of acceleromyography improved with preload and normalization? A comparison with mechanomyography. Anesthesiology. 2009;110:1261–70.
- Fuchs-Buder T, Claudius C, Skovgaard LT, et al. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51:789–808.
- Murphy GS, Szokol JW, Avram MJ, et al. Comparison of the TOFscan and the TOF-Watch SX during Recovery of Neuromuscular Function. Anesthesiology. 2018;129:880–888.
- Motamed C, Kirov K, Combes X, Duvaldestin P. Comparison between the Datex-Ohmeda M-NMT module and a force-displacement transducer for monitoring neuromuscular blockade. Eur J Anaesthesiol. 2003;20:467–469.
- Engbaek J, Ostergaard D, Viby-Mogensen J, Skovgaard LT. Clinical recovery and train-of-four ratio measured mechanically and electromyographically following atracurium. Anesthesiology. 1989;71:391–395.
- Kopman AF. The relationship of evoked electromyographic and mechanical responses following atracurium in humans. Anesthesiology. 1985;63:208–211.
- Harper NJ, Bradshaw EG, Healy TE. Evoked electromyographic and mechanical responses of the adductor pollicis compared during the onset of neuromuscular blockade by atracurium or alcuronium, and during antagonism by neostigmine. Br J Anaesth. 1986;58:1278–1284.
- Engbaek J. Monitoring of neuromuscular transmission by electromyography during anaesthesia. A comparison with mechanomyography in cat and man. Dan Med Bull. 1996;43:301–316.
- Kern SE, Johnson JO, Orr JA, Westenskow DR. Clinical analysis of the flexor hallucis brevis as an alternative site for monitoring neuromuscular block from mivacurium. J Clin Anesth. 1997;9:383–387.
- Veiga Ruiz G, Garcia Cayuela J, Orozco Montes J, et al. Monitoring intraoperative neuromuscular blockade and blood pressure with one device (TOF-Cuff): a comparative study with mechanomyography and invasive blood pressure. Rev Esp Anestesiol Reanim. 2017;64:560–567.
- Dahaba AA, Bornemann H, Holst B, Wilfinger G, Metzler H. Comparison of a new neuromuscular transmission monitor compressomyograph with mechanomyograph. Br J Anaesth. 2008;100:344–350.
- Krijtenburg P, Honing G, Martini C, et al. Comparison of the TOF-Cuff® monitor with electromyography and acceleromyography during recovery from neuromuscular block. Br J Anaesth. 2019;122:e22–e24.
- Renew JR, Hex K, Johnson P, et al. Ease of application of various neuromuscular devices for routine monitoring. Anesth Analg. 2021;132:1421–1428.
- Todd MM, Hindman BJ, King BJ. The implementation of quantitative electromyographic neuromuscular monitoring in an academic anesthesia department. Anesth Analg. 2014;119:323–331.