Anesthesiology News
Vice Chair of Academic Affairs
Department of Anesthesiology
Boston University School of Medicine
Boston, Massachusetts
Director, SICU East Newton Campus
Department of Anesthesiology
Boston University School of Medicine
Boston, Massachusetts
One source of resistance to the establishment of guidelines for monitoring neuromuscular function is the mistaken belief that patients do not experience clinically significant adverse outcomes related to residual neuromuscular blockade. The weight of the evidence, however, suggests that the opposite is true.
Case
A 47-year-old man was brought to the operating room for a laparoscopic cholecystectomy. General anesthesia was induced with propofol and fentanyl. Succinylcholine was administered in anticipation of endotracheal intubation. As the anesthesiologist performed direct laryngoscopy, the patient regurgitated clear gastric contents. Aspiration of the oral cavity with a suction cannula was immediately performed; however, some contents migrated into the trachea. A nerve stimulator was not used, and the patient did not exhibit the characteristic fasciculations after the administration of succinylcholine.
In retrospect, had the anesthesiologist evaluated neuromuscular function after the administration of the paralytic, it would have been possible to determine not only the onset of action of the drug but also the intensity of the neuromuscular block. This case highlights one of the complications that can arise from failure to assess neuromuscular function during the administration of neuromuscular blocking agents.
Any review of assessing neuromuscular function after administration of neuromuscular blocking agents should mention the contributions of French physiologist Claude Bernard. A decade after the introduction of ether anesthesia, his experiments in the 1850s helped unravel how neuromuscular blocking agents (NMBAs) produce paralysis. Using frogs, Bernard ligated the main artery in one of their hind legs while maintaining perfusion to the contralateral legs. The sciatic nerves were dissected and isolated, keeping them intact. Curare was then administered under the frog’s skin. Electrical stimulation of the sciatic nerve in the extremity with intact blood flow did not elicit muscular contractions. In contrast, stimulation of the nerve in the leg with a ligated artery triggered a contraction. Furthermore, direct stimulation of the muscle on either leg also resulted in contractions.
These experiments shed light on the mechanism of action of NMBAs and led to the eventual understanding of the neuromuscular junction.1,2 Bernard used electrical stimulation in his experiments almost 170 years ago. Today, this remains the only reliable method for monitoring neuromuscular function after the administration of paralytics in clinical practice.
The neuromuscular function of every patient should be monitored during the administration of NMBAs. When patients are conscious, evaluation of muscle strength using clinical assessments is possible. However, these assessments are subject to interpretation and do not accurately predict complete recovery of neuromuscular function.3,4 Typically, clinicians use electrical nerve stimulation and the resulting muscle contractions to monitor neuromuscular blockade.
For decades, anesthesia providers were taught to assess the adequacy of reversal of NMBAs using clinical signs, such as head lift, grip strength, leg lift, and negative inspiratory pressure. Today, we know that these tests are inadequate.5 Clinical management guided by subjective assessments can contribute to complications, such as hypoventilation and aspiration.6 None of the currently used clinical signs require sufficient muscle function to detect residual neuromuscular weakness, and consequently should not be trusted to make clinical decisions. However, there are situations in which clinical assessment of neuromuscular function can be useful, such as when neuromuscular monitoring devices malfunction or are unavailable.
The Association of Anaesthetists of Great Britain and Ireland recommends monitoring neuromuscular function during induction, maintenance, and termination of anesthesia.7 These guidelines state that “a measure of neuromuscular block, using a peripheral nerve stimulator (PNS), is essential for all stages of anaesthesia when neuromuscular blockade drugs are administered.” Furthermore, they advocate for the replacement of a qualitative PNS with neuromuscular monitoring devices capable of objective, quantitative analysis.
Although enthusiasm for establishing guidelines for monitoring neuromuscular function is increasing in the United States, the American Society of Anesthesiologists has not opined on this issue.6 One source of resistance to the establishment of guidelines for monitoring neuromuscular function is the mistaken belief that patients do not experience clinically significant adverse outcomes related to residual neuromuscular blockade.6 The weight of the evidence, however, suggests that the opposite is true.8-11
This review concentrates on the methods employed by anesthesia providers in the clinical setting to assess neuromuscular function after the administration of NMBAs. Considering the lethality of these drugs, anyone dedicated to the administration of anesthetics should be familiar with the use of nerve stimulators and how to monitor neuromuscular function.
Normal Neuromuscular Transmission
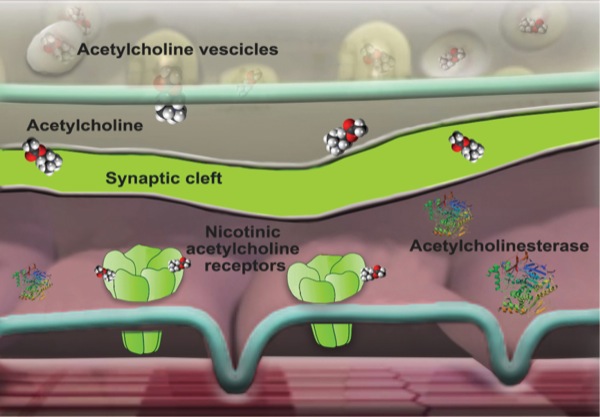
The administration of NMBAs demands an understanding of the basic principles of normal neuromuscular transmission. The neuromuscular junction consists of the motor nerve terminus, the postsynaptic muscle end plate, and the intervening gap known as the synaptic cleft. An action potential reaching the motor nerve terminus triggers the release of acetylcholine from the synaptic vesicles. Acetylcholine then rapidly diffuses across the gap to the postsynaptic end plate, where nicotinic acetylcholine receptors convert the chemical signal into an electrical impulse, causing depolarization in the postsynaptic membrane. Excitation–contraction coupling results in immediate muscle contraction. The action of acetylcholine is terminated by means of dissociation from receptors, passive diffusion away from the end plate, and ester hydrolysis by acetylcholinesterase (Figure 1).
Why Monitor Neuromuscular Function?
There are many reasons why monitoring neuromuscular function is essential for patient safety when paralytics are used. Fasciculations after the administration of succinylcholine are frequently used by clinicians as an indication of the drug’s onset of action. However, not all patients exhibit fasciculations, particularly cachectic patients or those being treated with magnesium sulfate.12,13
It is also possible for the medication to not be injected into the patient if there is an inadvertent IV line disconnection. The drug may have lost potency if expired or if not stored at an adequate cool temperature. Furthermore, in patients with very slow circulation, it is conceivable for succinylcholine to be hydrolyzed to a significant extent before it reaches the muscle end plate.14,15 The duration of action of succinylcholine varies greatly. In inherited or acquired cholinesterase abnormalities, such as pseudocholinesterase deficiency and severe protein malnutrition, succinylcholine’s effects may last much longer than expected.16
For these reasons, monitoring of neuromuscular function is required when succinylcholine is used, despite its rapid onset and short duration of action. When using non-depolarizing drugs, monitoring neuromuscular function measures the intensity of the block and, most importantly, helps assess the adequacy of block reversal.
Monitoring of Neuromuscular Function
Present State of Neuromonitoring
As discussed previously, although subjective assessments of neuromuscular function have been used for decades, they are unreliable. Consequently, there is impetus to supersede them with the use of objective, quantifiable information from neuromonitoring devices.7 These devices can provide both qualitative information in the form of visible muscle twitch and quantitative real-time measurements of neuromuscular function. Despite widespread availability, PNS and neuromuscular monitors are not used by a significant percentage of clinicians, and many continue to evaluate the patient’s neuromuscular function based on subjective signs and assessments alone.17
As neuromuscular monitors become more widely available, the need to display and record the data they generate is increasing. Ideally, graphics and values, such as those related to train-of-four (TOF) monitoring, should appear in the same screen as the pulse oximetry plethysmograph and capnography waveforms. The data generated by neuromuscular monitors should be integrated with anesthesia information management systems, similar to the data generated by other monitoring devices.
Sites of Nerve Stimulation
Although multiple nerves can be stimulated to assess neuromuscular function, the ulnar and facial nerves are used most frequently. Ulnar nerve stimulation triggers contraction of the adductor pollicis muscle, moving the thumb. This is convenient because the adductor pollicis is not adjacent to the stimulation site, minimizing the possibility of direct muscle stimulation. The adductor pollicis muscle can be monitored by placing stimulating electrodes along the ulnar nerve on the forearm. The distal negative electrode should be placed 2 cm proximal to the wrist crease, and the positive electrode should be placed approximately 4 cm proximal to the negative electrode along the ulnar nerve.
With the advent of minimally invasive surgery, which frequently requires the patient’s arms to be tucked by the side, access to the upper extremities is not always possible. In these situations, the facial nerve can be stimulated to monitor contraction of the orbicularis oculi and corrugator supercilii muscles. Alternatively, the flexor hallucis muscle can be monitored by stimulating the tibial nerve. The electrodes should be placed posterior to the medial malleolus to monitor flexion of the big toe.
PNS and Monitors
Administration of NMBAs and their subsequent effects on nerve conduction should be assessed with a PNS. The PNS is designed to deliver an electrical impulse to a peripheral nerve via electrodes in order to evoke a motor response. The electrical impulse should be supramaximal at a duration of 0.1 to 0.2 ms and an amplitude of 50 to 60 mA.18 Clinicians can subjectively assess the degree of motor blockade by visual and tactile methods.
Subjective assessments of neuromuscular function, such as head lift, do not exclude residual neuromuscular block. There is consensus in the literature that objective methods to evaluate neuromuscular function during the administration of NMBAs is superior to qualitative assessments.
Quantification of the degree of muscle relaxation induced during neuromuscular blockade can be performed using specialized nerve stimulators with built-in monitoring capabilities, such as acceleromyography, electromyography, kinemyography, and mechanomyography.
Acceleromyography is based on the direct relationship of acceleration to muscle contraction during nerve stimulation. A diminished muscle contraction from neuromuscular blockade reduces stimulated muscle acceleration, which can be quantified in real time. An accelerometer probe can be easily affixed to the distal tip of the thumb, eyebrow, or eyelid to detect contractions of the adductor pollicis, corrugator supercilii, and orbicularis occuli, respectively. This monitoring technique is gaining popularity and replacing the older, more cumbersome monitoring technologies, described below.
Electromyography detects and analyzes electrical signals from a stimulated muscle. This can be performed using stimulating electrodes along the ulnar nerve distribution and sensing electrodes over the adductor pollicis muscle group.
The analysis of muscle movement or contraction in response to an electrical signal during induced stimulation is called kinemyography. A common location for measurement is the hand, where a flexible mechanical sensor is strapped between the thumb and index finger. The resultant movement from thumb adduction during ulnar nerve stimulation is measured and analyzed.
Mechanomyography measures the isometric force generated during muscle contraction during peripheral nerve stimulation. The setup is typically cumbersome, requiring wrist fixation and a strapped thumb force transducer to measure adduction contraction force. Mechanomyography is rarely used in clinical practice.19
Nerve Stimulation Patterns and Corresponding Muscle Response (Table)
Single Twitch Stimulation
Single twitch (ST) stimulation is a muscular contraction elicited in a non-paralyzed patient when a single electrical stimulation is applied with a PNS at 0.1 or 0.15 Hz. The result is a visible twitchonce every 10 or 6.7 seconds, respectively. Each twitch has an expected amplitude in a non-paralyzed patient. The amplitude of each continuous ST will diminish with the administration of a NMBA. This change is theorized to correlate with postsynaptic nicotinic receptor block produced by the NMBA. ST stimulation during neuromuscular blockade, however, cannot be used to determine the class or type of NMBA administered. A specific clinical use of ST stimulation is the assessment of a post-tetanic twitch count (PTC).
TOF Stimulation
A TOF stimulation is a sequence of 4 consecutive electrical stimuli (i.e. T1-T4) delivered via a PNS at 2 Hz (every 0.5 second). All 4 twitches will evoke a consistent muscle contraction amplitude in a non-paralyzed patient. The number of observed muscle contractions (0 to 4) during a TOF stimulation is called a TOF count (TOFC). The TOF ratio (TOFR) is measured by the amplitude of T4 divided by T1. The TOF stimulation, and its associated TOFC and TOFR, is arguably the most practical application of neuromuscular stimulation during the use of NMBAs.
In a superficial depolarizing block, the TOFC is 4 of 4, with amplitudes equally reduced across all stimuli. The subsequent TOFR will be 1.0. In a non-depolarizing block, the TOFC will progressively decrease to less than 4. This is marked by an initial reduction in the amplitude of the last twitch (T4) to a gradual reduction in the amplitude of T3 through T1. This gradual reduction of amplitude height from T4 to T1 with increasing block depth in non-depolarizing agents, but not in depolarizing agents, is called a TOF fade. The TOFR will be less than 1.0 in a non-depolarizing block.
Subjective determination of TOFR is made by first assessing a baseline TOFC in a non-paralyzed patient, followed by an assessment of strength and presence of all 4 evoked twitches when a non-depolarizing block is induced. A subjective TOFR is provided by comparing the total twitches detected with the expected twitch count. For example, TOFC can range from 4/4 to 0/4, depending on the block depth. The challenge for clinicians is that after a TOFC of 4/4, a subjective assessment of fade cannot be reliably detected. Quantitative monitors allow clinicians to assess TOFR objectively during a depolarizing or non-depolarizing block. This is important given that a TOFR of 0.9 is the minimum ratio at which any neuromuscular block weakness can be excluded before proceeding to tracheal extubation.20
Double Burst Stimulation
A double burst stimulation (DBS) is a coupled sequence of high-frequency stimuli at 50 Hz delivered 750 ms apart. Each DBS is delivered as either 3-and-3 stimuli (DBS3,3) or 3-and-2 (DBS3,2) stimuli. The near-tetanic stimulation employed during DBS results in significantly stronger evoked muscle contractions than TOF stimulation in a non-paralyzed patient. For this reason, DBS may improve subjective assessment of residual neuromuscular paralysis over TOF stimulation.
Tetanic Stimulation
A tetanic stimulation (TET) is a single, high-frequency stimulation delivered at 50 Hz for 5 seconds. A strong muscle contraction is evoked and sustained without fade or post-tetanic potentiation in a non-paralyzed patient. Particular caution should be used when employing TET. TET should never be delivered to an awake patient, as it can cause severe pain. Repeated TET delivery over 50 Hz can cause the appearance of fade due to muscle fatigue and not neuromuscular blockade. Repeated TET deliveries less than 2 minutes apart can hasten detected recovery at the site of stimulation or produce post-tetanic potentiation despite a systemically deeper level of blockade—giving clinicians a false sense of overall neuromuscular recovery. Acting on this incorrect assessment may compromise patient safety if clinicians unnecessarily administer more NMBAs. The utility of TET is best exemplified during its co-application to a PTC.
Post-Tetanic Twitch Count
A PTC assesses the number and amplitude of evoked muscle contractions after a delivered TET at 50 Hz for 5 seconds, followed immediately by continuous ST at 1 Hz for 20 seconds. Its clinical application is limited to estimating the time expected for the first detectable twitch of a TOFC during deep non-depolarizing neuromuscular blockade.
Complications of Monitoring Neuromuscular Function
Although nerve stimulators are very safe devices, complications can occur, ranging from transient pain during nerve stimulation to respiratory failure resulting from misinterpretation of the information they provide. Other complications include muscle pain resulting from overstimulation and pressure injuries caused by the electrodes. It is also possible for nerve stimulators to cause burns if there is an equipment malfunction and interference with an ECG or implanted pacemaker.
Sugammadex
Although the introduction of sugammadex (Bridion, Merck), a selective aminosteroid-binding reversal agent, facilitates managing neuromuscular blockade for surgical procedures, monitoring the neuromuscular block and its reversal is still required.17,21 When sugammadex is used without neuromuscular function monitoring, there is a 10% incidence of residual neuromuscular blockade.22 Furthermore, its widespread and routine use is limited by cost, and it may not adequately reverse the effects of benzylisoquinoline drugs, such as cisatracurium.17
Conclusion
Because subjective assessment is not reliable, we strongly recommend quantitatively monitoring neuromuscular function when NMBAs are used. Routine monitoring should ideally be performed with objective measurement techniques, such as acceleromyography. Monitoring neuromuscular function poses minimal risk to patients and may avoid serious complications associated with incorrect assessment of neuromuscular blockade.
References
- Black J. Claude Bernard on the action of curare. BMJ. 1999;319(7210):622.
- Cousin MT. Vulpian and not Claude Bernard first proposed the hypothesis of the motor end-plate as the site of action of curare. Anesthesiology. 2002;97(2):527-528.
- Fortier L-P, McKeen D, Turner K, et al. The RECITE Study. Anesth Analg. 2015;121(2):366-372.
- Aytac I, Postaci A, Aytac B, et al. Survey of postoperative residual curarization, acute respiratory events and approach of anesthesiologists. Braz J Anesthesiol. 2016;66(1):55-62.
- Cammu G, De Witte J, De Veylder J, et al. Postoperative residual paralysis in outpatients versus inpatients. Anesth Analg. 2006;102(2):426-429.
- Brull SJ, Kopman AF. Current status of neuromuscular reversal and monitoring. Anesthesiology. 2017;126(1):173-190.
- Checketts MR, Alladi R, Ferguson K, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2016;71(1):85-93.
- Murphy GS, Szokol JW, Avram MJ, et al. Residual neuromuscular block in the elderly. Anesthesiology. 2015;123(6):1322-1336.
- Kiekkas P, Bakalis N, Stefanopoulos N, et al. Residual neuromuscular blockade and postoperative critical respiratory events: literature review. J Clin Nurs. 2014;23(21-22):3025-3035.
- Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111(1):120-128.
- Murphy GS. Residual neuromuscular blockade: incidence, assessment, and relevance in the postoperative period. Minerva Anestesiol. 2006;72(3):97-109.
- Srinivasan PR, Patwardhan V. Plasma-esterase and plasma-lipase levels in nutritional oedema syndrome (kwashiorkor). Lancet. 1952;2(6740):864-866.
- Stacey MR, Barclay K, Asai T, et al. Effects of magnesium sulphate on suxamethonium-induced complications during rapid-sequence induction of anaesthesia. Anaesthesia. 1995;50(11):933-936.
- Donati F. Onset of action of relaxants. Can J Anaesth. 1988;35(3 pt 2):S52-S58.
- Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110(5):1176-1181.
- Durant NN, Katz RL. Suxamethonium. Br J Anaesth. 1982;54(2):195-208.
- Naguib M, Brull SJ, Johnson KB. Conceptual and technical insights into the basis of neuromuscular monitoring. Anaesthesia. 2017;72(suppl 1):16-37.
- Kopman AF, Lawson D. Milliamperage requirements for supramaximal stimulation of the ulnar nerve with surface electrodes. Anesthesiology. 1984;61(1):83-85.
- Fuchs-Buder T, Claudius C, Skovgaard LT, et al. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51(7):789-808.
- Thilen SR, Bhananker SM. Qualitative neuromuscular monitoring: how to optimize the use of a peripheral nerve stimulator to reduce the risk of residual neuromuscular blockade. Curr Anesthesiol Rep. 2016;6:164-169.
- Abad-Gurumeta A, Ripollés-Melchor J, Casans-Francés R, et al. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia. 2015;70(12):1441-1452.
- Kotake Y, Ochiai R, Suzuki T, et al. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg. 2013;117(2):345-351.


Leave a Reply
You must be logged in to post a comment.