The supraglottic airway device (SAD) has earned a well-regarded position in both anesthesia care and emergency airway management. Its prominent position in various societal algorithms for rescue airway management is testimony to its ubiquitous availability, its relative ease of use, and relatively high success rate under adverse clinical conditions. Providing rescue ventilation/oxygenation and serving as an intubation conduit for both emergent and elective patients are distinct advantages.1-8 Employing an intubation introducer such as a flexible fiber-optic bronchoscope (FFB) through a SAD to facilitate tracheal intubation is a valuable rescue technique that melds several individual devices into a reliable rescue tactic that can be made readily available.8-15
Higgs et al described the hallmarks of an ideal technique to achieve intubation when conventional methods have failed16:
- equipment that is universally available for immediate control of the airway;
- “an upper airway device which maintains airway patency while facilitating tracheal intubation”;
- universal and transferable skills that are easily learned;
- the ability to maintain airway patency and supplemental gas exchange until the trachea is secured; and
- the ability to visualize the advancement of a trachea tube via the device.
Although this job description for the SAD predated the rapid proliferation and widespread availability of video laryngoscopy (VL), it remains accurate and steadfast in delineating the important role the SAD serves in airway management, both inside and outside the OR.
Alternatives to the classic SAD design specifically intended to facilitate blind advancement of a trachea tube have been quite successful in serving as rescue devices in the OR and non-OR areas (NORA) locations, including the emergency department and emergency medical services and for medical flight crew activities.8-15 The Intubating models of the LMA family (ILMA, LMA Fastrach; Teleflex) and other similar SAD products serve an important role by affording blind or FFB-assisted intubation with a relatively high success rate. The ability of the operator to reliably intubate the trachea blindly serves as a distinct advantage when fiber-optic equipment or FFB-skilled personnel are not available, or when visualization is impeded by secretions, soilage, trauma or edema.
SAD models specifically designed for blind intubation are far fewer in number. Blind SAD intubation via the LMA Classic (Teleflex), which was the original laryngeal airway mask, is unreliable.17-23 Despite effective ventilation and oxygenation, the LMA Classic may be malpositioned within the airway. Effective ventilation does not ensure adequate positioning of the LMA Classic. Cuff malposition, epiglottic down-folding and arytenoid impingement may deflect reliable delivery of a tracheal tube to the laryngeal inlet.24
Blind passage of an airway introducer or a bougie via the SAD is another option, but each has its own limitations and difficulties. Alternatively, passing both a small-caliber FFB alongside a bougie, within the SAD lumen, may allow visual advancement of the bougie tip into the trachea. These approaches should be pursued only if the more successful FFB options (described below) do not exist.
It is recommended that when utilizing the standard SAD models, such as the LMA Classic, fiber-optic visualization should be deployed when attempting intubation. There are essentially two basic choices for advancing an endotracheal tube (ETT) via the SAD:
- The ETT is advanced via the SAD lumen directly and then fiber-optically advanced into the trachea.
- An FFB–airway catheter combination is advanced via the SAD lumen and into the trachea, leaving the airway catheter in place, then removing the SAD, which is followed by ETT advancement over the indwelling airway catheter.
Visually advancing a smaller-caliber ETT via the SAD with FFB assistance (choice 1) is certainly acceptable if there is a clear understanding of a proper pairing of the SAD with the ETT. The luminal caliber of the SAD and its pairing with an appropriately sized ETT should be understood before deploying this technique in the emergency rescue setting. Passing an ETT via the LMA Classic is limited in several respects:
- The ETT caliber, and hence its length, may limit the depth of the ETT tip and cuff in relation to the glottis.
- Once intubated, the LMA Classic may be quite difficult to remove over the indwelling ETT.
- Leaving the ETT and LMA Classic in place for the duration of a surgical procedure may be appropriate, but this is not satisfactory for an ICU or ward rescue intubation.
- Exchange of the ETT and LMA Classic over an airway exchange catheter may be required.
The second option (choice 2) would be to pair the FFB with an intubation guide (Aintree Intubation Catheter [AIC], Cook Medical). The AIC is essentially the Cook Medical 19 Fr Airway Exchange Catheter, shortened to 57 cm long, with a roomier internal diameter to accommodate the passing of a standard small adult FFB. This ventilation-exchange bougie, with properly sized internal (4-mm) and external (6.3-mm) diameters, was designed to fit onto the insertion cord of the bronchoscope, allowing the 3-cm distal tip to be free and unsheathed, maintaining its flexible maneuverability. The practitioner can then maneuver the FFB and AIC through the laryngeal inlet. The AIC is then slid off the FFB and advanced into the trachea. Once the AIC is placed in the trachea, the FFB and SAD may be carefully removed, with attention to maintaining the AIC position so it does not migrate too distally or too proximally. Then an ETT is advanced over the AIC as one would when using an airway catheter or a bougie. The large caliber of the AIC affords advancement of a 7.0–mm or larger ETT.
The ILMA and other similar SAD models are designed to afford both blind and FFB-assisted intubation. Fiber-optic skills vary widely among anesthesia practitioners, which is why some routine practice to maintain confidence and competency with the FFB should be pursued. Advancing the FFB via the laryngeal mask (LM) airway to reach the laryngeal inlet is less onerous than asleep or awake FFB intubation. The SAD offers a tremendous supplemental advantage as an intubation conduit. Bronchoscopy via the SAD affords free maneuvering of the FFB unimpeded past the majority of the airway (oral cavity, oro-hypopharynx), leading to only distal airway manipulation of the FFB. Some have deemed these procedural skills to be reliable and “low skilled.”16 Certainly, the SAD removes the upper airway pathway from the FFB challenge, as maneuverability past this portion of the airway may be the most significant obstacle in bronchoscopy-assisted intubation. The AIC can be used with either an LMA Classic or ILMA-type SAD.
Although the LMA Classic style of SAD is universally practiced as a ventilating device, its role as an intubation conduit requires knowledge and experience in order for it to act as a reliable rescue adjunct. Adapting the FFB and LMA Classic combination or the blind ILMA technique under emergent conditions without prior review or practice of the procedure should be discouraged. Regardless of which type of SAD is used, basic familiarity with the concept and procedural steps can be easily reviewed with online video modules and schematic lessons. Practice on a mannequin with the assistance of an experienced instructor followed by its use under elective OR conditions should be encouraged. The blind ILMA technique requires an orderly approach, execution of distinct steps and an understanding of how to troubleshoot difficulties. The blind ILMA technique is a distinct skill with its own learning curve.16
The author has noticed that its popularity, as a go-to rescue device in the OR and NORA, has declined considerably since the advent and widespread deployment of VL. However, at our institution, the SAD remains the most common backup rescue device for VL difficulties and failures inside and outside the OR. The skill set required for either the blind method (ILMA) or FFB-assisted method (LMA Classic/ILMA) is reliant on regular practice and ongoing efforts to maintain competency.
Utilizing an SAD following a failed intubation has a relatively high success rate for achieving effective ventilation. Blind ETT advancement by a skilled practitioner has a high success rate after three attempts in the ILMA model.8,10,13 If the FFB and AIC combination is chosen and a period of apnea can be safely pursued, passing the FFB and AIC through either LMA model can be pursued once the circuit or reservoir bag has been removed. Otherwise, a bronchoscopic adapter may be placed on the LMA model to allow continued delivery of oxygen as well as the luxury of providing positive pressure ventilation during the intubation procedure. Ongoing oxygenation via the “closed circuit” is certainly advantageous. Moreover, the ability to apply positive pressure ventilation via the SAD to pressurize airway structures may assist with lateralizing edematous periglottic anatomy and afford the endoscopist an improved view of the laryngeal inlet. This could be a lifesaving maneuver.
The impressive success rate of intubating blindly via the ILMA was established by Ferson et al and others.8,13 However, the practitioners performing airway management in those reports were seasoned and well-practiced individuals. The near-perfect track record of its success, as reported in these publications, reflects proper preparation, procedural review and ongoing experience of the practitioners with this airway adjunct. These outstanding results would not likely be replicated by the average, less experienced anesthesia provider. Berkow et al and others reported a high intubation success rate, incorporating the LMA Classic, AIC and FFB method for difficult airway patients in the OR.9-12 To date, reports using this rescue method in remote NORA locations are sparse. Despite its ubiquitous presence in airway management algorithms for rescue ventilation and as an intubation conduit, its success rate in NORA emergency encounters has not been firmly established.
Review of the Hartford Hospital Emergency Intubation Database
A quality improvement database of emergency intubations in NORA locations performed by the Department of Anesthesiology at Hartford Hospital, in Farmington, Conn., between 1990 and 2018 was reviewed after institutional review board consent. Written informed consent was waived with approval to investigate SAD use for non-OR emergency airway management, excluding intubations for cardiac arrest. The database contained a total of 18,832 patient encounters. The airway management team included either an attending anesthesiologist alone or one or more supervised anesthesia resident trainees, with certified registered nurse anesthetist team members practicing during evening/night and weekend hours.
The emergent use of the SAD played three roles:
- as an intubation conduit after conventional intubation attempts or VL were unsuccessful;
- as a ventilation/oxygenation bridge to another management technique; and
- as an initial (primary) management device (Table 1).
| Table 1. SAD Airway Management Roles (N=1,461) |
| 1. Conventional DL was unsuccessful (n=1,046) |
| 2. DL + bougie was unsuccessful (n=147) |
| 3. Primary VL use was unsuccessful (n=113) |
| 4. DL then VL use were unsuccessful (n=94) |
| 5. DL + bougie then VL were unsuccessful (n=23) |
| 6. SAD served as primary management device (n=38) |
| Primary, initial device used for airway management.
DL, direct laryngoscopy; SAD, supraglottic airway device; VL, video laryngoscopy
|
The database was reviewed to determine the number of attempts to attain satisfactory SAD ventilation (LMA Classic and ILMA); the method of intubation, if any (FFB-assisted or blind); the number of attempts needed for SAD-assisted intubation; and the rescue techniques incorporated for SAD failures.
Overall, 1,461 patients underwent a SAD intervention (LMA Classic/ILMA) during NORA emergency airway management over a 27-year period (overall, 7.8%; Table 1). As previously mentioned, this does not include any cases where a SAD was used to assist airway management during a NORA case presenting as a cardiac arrest. Conventional direct laryngoscopy (DL) was clearly the largest benefactor of SAD rescue from over 14,000 DL and DL plus bougie interventions. The SAD served as a rescue device for primary VL difficulty/failure as well as a bridge when VL was utilized in the role of rescue adjunct. The SAD had a successful role as a primary management adjunct in a small number of patients (n=38) with a known or suspected difficult airway. Moreover, the SAD served as a ventilation/oxygenation bridge in a variety of emergency airway situations when no attempts were pursued to secure the airway via SAD-assisted intubation (Table 1).
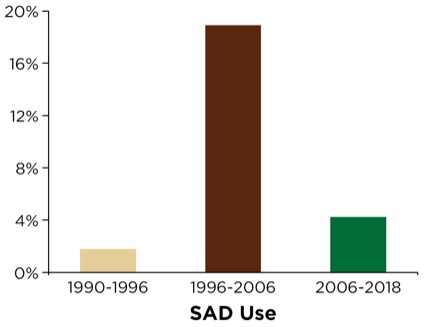
The SAD was used sparsely from 1990 to 1996 (60 deployments in 3,289 cases; 1.8%) due to its relative lack of availability in remote locations outside the OR (Figure 1). The department and institution, commencing with the publication of the American Society of Anesthesiologists’ (ASA’s) difficult airway algorithm, coupled with its comprehensive airway management review and recommendations,2 subsequently organized and distributed difficult airway carts (DACs) within OR and PACU areas as well as strategic and high-traffic NORA locations throughout the institution (e.g., radiology, gastroenterology suite, emergency department, all ICU areas and the cardiac catheterization suites). Additionally, portable/mobile suitcases containing adjunct airway devices and equipment were deployed and carried by the anesthesia stat airway team. The anesthesia stat airway team was primarily responsible for NORA airway management throughout the institution and served as backup and consultants for the emergency department. These suitcases were brought to the patient’s bedside, and contained a variety of primary and secondary SADs as well as other adjuncts, as suggested by the ASA’s recommendations.
SAD use soon expanded substantially; that is, between 1996 and 2006, there were 964 deployments in 5,089 cases (18.9%) following efforts to ensure its ubiquitous availability in NORA settings (Figure 1). Later in 2006, the acquisition and dissemination of video laryngoscopic devices (GlideScope and the portable GlideScope Ranger, Verathon) ushered in the airway team’s transition and rapid embracement of being VL-capable for NORA emergency airway management. Review of the database following VL deployment revealed that SAD utilization underwent a substantial decline; between 2006 and 2018, there were 437 deployments in 10,423 cases (4.2%; Figure 1).
SAD-Assisted Ventilation
Of the 1,461 emergency cases utilizing either of the SAD models (LMA Classic/ILMA), successful ventilation of the patient was impressive despite the emergency nature of the NORA airway intervention (Table 2). The ability to establish effective ventilation (typically limited to four attempts) with the SAD was substantial, at 95.1%, with 69.5% on the first attempt and 90.1% by the second attempt (reseating/changing LMA size/model). Overall ventilation successes with either LMA model were similar. The first-attempt success rate, success after two attempts, and the overall success rate for both LMA models were indistinguishable. Reseating or changing the size of the SAD was an attempted remedy for unsuccessful ventilation and oxygenation. Moreover, switching to another SAD model (LMA Classic→ILMA) occurred with relative frequency. The LMA Classic model was switched to the ILMA model in 73 patients, and the ILMA was switched to the LMA Classic in 34 cases. The need to switch models was associated with an increased failure rate for both SAD ventilation efforts (Table 2).
| Table 2. SAD Ventilation by Model (N=1,461) | ||||
| SAD Model | First-Attempt Success | First-/Second-Attempt Success | Overall Success | Failures |
|---|---|---|---|---|
| Overall | 69.5% | 90.1% | 95.1% | 4.9% |
| LMA Classic (Teleflex; n=381) | 72.6% | 92.0% | 96.2% | 3.8% |
| ILMA (Teleflex; n=973) | 70.3% | 93.4% | 97.4% | 2.6% |
| ILMA→LMA Classic (n=34) | 26.5% | 53% | 67.6% | 32.4% |
| LMA Classic→ILMA (n=73) | 45.2% | 65.7% | 80.8% | 19.2% |
| ILMA, Intubating LMA | ||||
As noted, the ventilation failure rates were quite low for both SAD models. However, failure of one model that led to transitioning to another model was met with a considerable increase in difficulty and unsuccessful deployment, despite reseating or changing the LMA size or model. Both ventilation and subsequent intubation via the alternative SAD was met with a much lower success rate for the airway team. Ventilation failures were rescued by a variety of methods, as outlined in Table 3. More than one-half of the SAD ventilation failures led to placement of a secondary SAD (Combitube). This deployment afforded temporary rescue until another device or technique was used to secure the airway via tracheal intubation (described in more detail below).
| Table 3. SAD Ventilation Failures (N=72): Rescue Methods Deployed |
| DL (n=0) |
| DL + bougie (n=9) |
| VL (n=15) |
| FFB (n=11) |
| Combitube→DL (n=6) |
| Combitube→DL + bougie (n=6) |
| Combitube→FFB (n=4) |
| Combitube→VL (n=8) |
| Combitube→IAA (n=13) |
| DL, direct laryngoscopy; FFB, flexible fiber-optic bronchoscope; IAA, invasive airway adjunct (surgical airway); SAD, supraglottic airway device; VL, video laryngoscopy |
A smaller population (n=165) underwent successful SAD ventilation, but no tracheal intubation was attempted. Of these patients, SAD placement (ILMA, n=48; LMA Classic, n=117) afforded a “bridge” for ventilation/oxygenation purposes. The SAD supported oxygenation and ventilation, whereas another airway method/device was readied and then deployed. SAD placement between intubation attempts proved useful for supplementing oxygen delivery to those patients with an anticipated/suspected or known difficult airway. Another clinical situation leading to SAD rescue as a bridge was oxygen desaturation following induction associated with difficult mask ventilation. Moreover, serving as a bridge while performing an invasive airway access procedure (i.e., a surgical airway) or affording temporary support during efforts by the airway team for equipment acquisition of preparation of other rescue modalities proved vital (Table 4).
| Table 4. SAD Deployment as a Bridge (prior to intubation attempts/between attempts) | |
| Ventilation/Oxygenation Bridge | N=165 |
|
n=33 |
|
n=17 |
|
n=74 |
|
n=41 |
| DL, direct laryngoscopy; IAA, invasive airway adjunct (surgical airway); SAD, supraglottic airway device; VL,video laryngoscopy | |
SAD-Assisted Intubation
Discounting those patients who had failed SAD ventilation attempts (n=72) from the original 1,461 SAD encounters leaves the remaining patients eligible for SAD-assisted intubation (n=1,389). In addition, accounting for the patients who were managed without any SAD-assisted intubation attempts (n=165), the remainder (n=1,224) underwent attempts of tracheal intubation, either blindly (ILMA) or FFB-assisted (LMA Classic/ILMA). Successful intubation overall, by any method, was 91.7%. Specifically, any intubation attempt (blind or FFB-assisted) was successful on the first attempt (66.2%) or by the second attempt (87.5%) in the majority of patients (Table 5).
| Table 5. SAD Intubation by Model/Method | ||||
| SAD Model | First-Attempt Success | First-/Second-Attempt Success | Overall Success | Failures |
|---|---|---|---|---|
| Overall (N=1,224) | 66.2% | 87.5% | 91.7% | 8.3% |
| LMA Classic (Teleflex; FFB, 261) | 73.4% | 91.5% | 96.5% | 3.5% |
| ILMA (Teleflex; all, 890) | 62.3% | 91.3% | 94.8% | 5.2% |
| ILMA (blind, 696) | 62.6% | 91.2% | 95.7% | 4.3% |
| ILMA (FFB, 194) | 60.6% | 89.4% | 89.9% | 11.1% |
| FFB, flexible fiber-optic bronchoscope; ILMA, Intubating LMA; SAD, supraglottic airway device | ||||
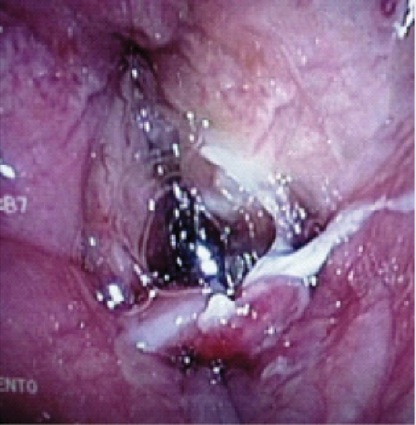
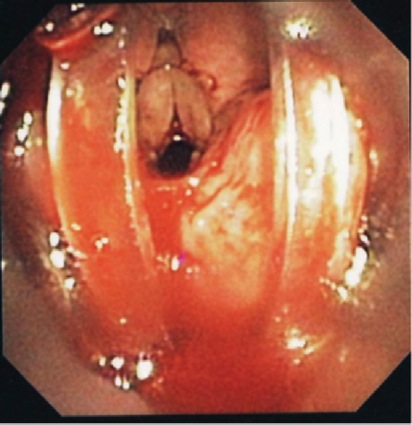
Use of the LMA Classic as an intubation conduit always employed FFB. Of the patients successfully ventilated via the LMA Classic (n=261), 96.5% (n=250) were successfully intubated by one of two methods: passing an AIC over the FFB (LMA Classic, AIC and FFB method, n=235; Figure 2) or passing a No. 6.0 ETT via the LMA Classic (LMA Classic, ETT and FFB method, n=15), which included a Mallinckrodt Microlaryngeal Tube (11 cases) and standard 6.0 ETT (four cases; Figures 3 and 4).
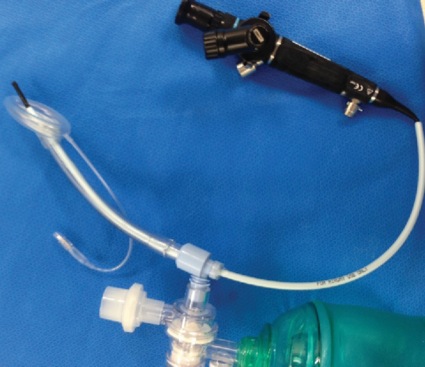
These 15 patients, who had the FFB-assisted intubation by having an ETT placed through the LMA Classic, required subsequent removal and exchange of the LMA Classic and ETT unit over an Airway Exchange Catheter (Cook Medical). Once the ETT was placed through the LMA Classic lumen, it was not deemed safe to attempt removal of the LMA Classic over the indwelling ETT. The primary problem is the tight fit between the ETT and the LMA Classic, leading to difficulty removing the LMA Classic while maintaining continued ETT positioning within the trachea. All LMA Classic and ETT exchanges were performed with laryngoscopic assistance combined with an airway exchange catheter to maintain access to the airway throughout the procedure, as is recommended by national guidelines. All 15 exchange cases were performed without complications. Secretions, altered anatomy, and periglottic swelling derailed 11 of 261 LMA Classic and FFB procedures due to poor visualization, inability to advance the AIC, AIC kinking or difficult ETT passage (Table 5).
As shown in Table 5, the airway team favored the ILMA over the LMA Classic, likely due to the former’s ability to blindly intubate with a reasonably high expectation of success. Immediate access to FFB equipment and a less soiled airway were likely factors in the decision-making process. Airway control using the ILMA weighted heavily toward the blind approach. The FFB-assisted advancement of the ETT via the ILMA was chosen by the airway team based on two clinical categories:
- elective use of the FFB as the method to pass the ETT via the ILMA (73% of the total FFB-ILMA cases; 95% FFB-assisted success rate); or
- rescue use of the FFB after difficult or unsuccessful blind ILMA intubation attempts (27% of the total FFB-ILMA cases; 82% FFB-assisted success rate).
Overall, the success rate for blind and FFB-assisted intubation via a SAD was impressive. It is particularly remarkable that within two attempts the overall success rate was in the 90% range given emergency clinical conditions. These results rival those trials in the elective setting in the OR for patients with normal and difficult airways (Table 5).
SAD intubation failures were rescued by a variety of methods, as outlined in Table 6. More than one-half of the SAD intubation failures led to an invasive airway adjunct (e.g., a surgical airway) to secure the airway. The key advantage of SAD support, despite the failure to intubate via the SAD, is that ongoing oxygenation and ventilation during performance of the surgical airway were key elements in improving patient care. A common rescue intervention for the SAD failures was a secondary SAD (Combitube), particularly prominent during the second time period (1996-2006), prior to VL deployment (see below). The secondary SAD afforded oxygenation and ventilation support while another device/technique was deployed to assist with the ultimate goal: trachea intubation. Use of VL after 2006 provided rescues for a significant number of SAD intubation failures.
| Table 6. Rescue Methods; SAD Intubation Failures (N=101) |
| DL (n=1) |
| DL + bougie (n=8) |
| VL (n=22) |
| SAD→IAA (n=51) |
| Combitube→DL (n=6) |
| Combitube→DL + bougie (n=5) |
| Combitube→FFB (n=5) |
| Combitube→VL (n=2) |
| Combitube→IAA (n=1) |
| DL, direct laryngoscopy; FFB, flexible fiber-optic bronchoscope; IAA, invasive airway adjunct (surgical airway); SAD, supraglottic airway device; VL, video laryngoscopy |
Elective SAD Utilization
Thirty-eight patients underwent elective SAD (33 ILMA; five LMA Classic) placement as the primary airway management method due to known or suspected difficult airway characteristics. Patient preparation for placement of the SAD included local anesthesia topicalization (n=8), propofol (n=8), midazolam (n=15) or etomidate (n=7). Spontaneous ventilation was maintained in all but three patients who received neuromuscular blocking agents. Each case established successful ventilation within three attempts. Thirty-six were successful within two attempts, and in two cases, three attempts. SAD-assisted intubation was successful in 36 of the 38 cases. The two SAD intubation failures underwent SAD conversion from the LMA Classic→ILMA. The majority of patients were managed with blind ILMA intubation, yet 12 patients were assisted with FFB-guided intubation (eight ILMA; four LMA Classic). The two cases of failed SAD-assisted intubation of the trachea were managed with VL rescue.
Application of Positive Pressure Ventilation
A significant number of the FFB-assisted SAD intubation failures were due to notable periglottic edema and anatomic distortion. Although SAD-assisted ventilation was successful, passage of the FFB was challenging due to signficant difficulty locating the laryngeal inlet. The application of positive pressure ventilation, in the presence of distorted, swollen and severe edematous periglottic tissues, can be interpreted as meaning that the glottis was patent. However, the periglottis tissues proved collapsible, and the laryngeal inlet was unrecognizable at atmospheric pressure. Hence, a closed circuit on the SAD was put to the test via a bronchoscopic adapter (Figures 3 and 4). The FFB and AIC could then be advanced through the bronchoscopic adapter while positive pressure ventilation was applied with ongoing oxygen delivery. Pressurization of the SAD closed circuit afforded lateralization of the edematous periglottic tissues and assisted immensely in visualization of the anatomic structures (Figure 5). Even minor tissue displacement by the applied pressurization afforded a quick glimpse of the pathway for the AIC, assisted by FFB placement. This proved successful in 13 of 18 patient encounters (9/13 LMA Classic and FFB cases and 4/5 ILMA and FFB cases; Figure 6).
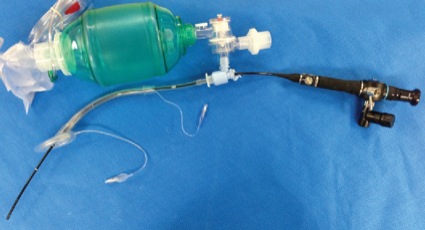

Secondary SAD Rescue Adjuncts
The airway team had a wide breadth of SAD models and sizes to choose from within the DAC and an ample selection from the portable suitcase. Both the LMA Classic and ILMA models are accompanied by the Supreme model from Teleflex, in an assortment of adult sizes. To further assist airway management, a SAD that offers airway control in an alternative fashion, such as an esophageal tracheal airway like the Combitube, is available in the DAC and suitcase. The Combitube and the more compact, single-cuffed esophageal tracheal airway device called the King LT (laryngeal tube; Ambu) could serve a valuable role as an alternative or secondary SAD because of its positioning in the airway, compared with an LMA-style SAD.
Overall, the utilization of a secondary SAD was relatively rare (n=65; ≈0.4%), but the majority of interventions (54/65) were following unsuccessful or unsatisfactory rescue use of the LMA Classic/ILMA. Deployment of the Combitube required additional adjuncts, as the ultimate goal was to control the airway with tracheal intubation in some form. The vast majority of these cases benefited greatly from secondary SAD support (e.g., establishment of oxygenation and ventilation) during which another airway adjunct was readied and then deployed. The most common post-Combitube interventions pursued with the hope of achieving tracheal intubation used DL alone, DL plus a bougie, an invasive airway adjunct (surgical airway), VL and FFB-assisted intubation (Table 7). Continuous oxygenation and ventilation support were provided via the Combitube during performance of a surgical airway.
| Table 7. Combitube Rescue Adjuncts | |
| Intubation Adjunct Utilized After Combitube Placement | Cases, n |
|---|---|
| DL | 7 |
| DL + bougie | 13 |
| FFB (around Combitube) | 6 |
| VL | 14 |
| IAA (surgical airway) | 13 |
| DL, direct laryngoscopy; FFB, flexible fiber-optic bronchoscope; IAA, invasive airway adjunct (surgical airway); VL, video laryngoscopy | |
SAD-Related Esophageal Intubation
Esophageal intubation during SAD intubation occurred relatively frequently (10.5%, or 129/1,224 cases). Nearly all of the esophageal intubations were during blind intubation attempts with the ILMA (92.2%, 119/129 cases). Ten esophageal intubations involved FFB-guided misplacement of the ETT (five LMA Classic; five ILMA). Fifteen ILMA patients experienced two esophageal intubations, each during blind intubation attempts.
Airway Soilage
The airway team cared for a significant number of patients with preexisting airway soilage, including watery or thick mucoid secretions, vomitus or blood. This soilage contributed to the level of difficulty of the laryngoscopy, leading to use of a SAD as a rescue adjunct. Airway soilage, despite aggressive efforts to suction the debris out and away from the airway, contributed to visualization difficulties when an FFB was used as an intubation aid via the SAD.
Despite this major obstacle, the success rate for FFB-assisted intubation remained impressively high. On arrival at the bedside, 229 of the 1,461 SAD patients were found to have significant soilage (i.e., vomitus or blood) in the airway, prompting vigorous suctioning prior to mask ventilation and/or intubation efforts (Table 8). These instances of airway soilage contributed to oxygenation and ventilation as well as visualization impairment during FFB-assisted intubation. A total of 27 of the 1,461 patients (1.8%) regurgitated prior to SAD insertion (during laryngoscopy), which contributed to 12 cases of tracheobronchial contamination (aspiration). There were no documented cases of regurgitation/aspiration during SAD support/manipulation of the airway (Table 8).
| Table 8. Airway Soilage: Preexisting Or During Emergency Airway Encounters | |
| Reason for Intubation | Patients |
|---|---|
| Preexisting blood in the airway | 136 |
| Preexisting vomitus in the airway | 93 |
| Moderate/significant secretions in the airway | 445 |
| Regurgitation/aspiration: initial management attempts | Regurgitation, 27; aspiration, 12 |
| Regurgitation/aspiration during SAD support | 0 documented |
| SAD, supraglottic airway device | |
Miscellaneous Issues
Following ILMA-assisted intubation of the trachea, removal of the ILMA over the indwelling ETT was complicated by tracheal extubation in nine cases. Prompt airway control was achieved with ILMA replacement (n=7) or VL intubation (n=2). It is imperative that the airway team remains together following successful intubation via the SAD. Explantation of the ILMA over the indwelling ETT seems like an easy Seldinger technique. However, it is my contention that even when practicing ILMA use on a mannequin or in an elective OR setting, removal of the ILMA over the ETT can be tricky, even in experienced hands. Thus, explantation of the ILMA in a NORA location in a difficult airway patient demands and warrants focus and vigilance. Accidental extubation during explantation may be life-threatening and lend to multiple hemodynamic and airway-related complications, as well as loss of the airway.
On occasion, the immediate post-intubation period is fraught with heart rate alterations, rhythm disturbances, hemodynamic perturbations, oxygenation and ventilation inequities, and sedation/analgesia concerns. These events accompany even a straightforward patient with first-pass success who required minimal airway management finesse. Combine these disruptions with a newly acclaimed difficult airway patient who just underwent five intubation attempts involving three different devices and experienced an episode of deep oxygen desaturation. Faced with this scenario, it may be wise to delay SAD explantation until the patient arrives in the ICU, is appropriately resuscitated, is hemodynamically stable, is properly sedated, and is somewhat returned to his or her critically ill baseline. Having said this, the database noted several cases of delayed SAD removal (two to 24 hours) and one case where SAD removal occurred at approximately the 36-hour mark (Table 9). These actions, with good intentions and patient safety in mind, would lead to a second airway procedure (SAD removal). On the one hand, the patient’s hemodynamics and ventilation/oxygenation status may have been stabilized, improved or optimized, yet the time delay afforded worsening of his or her airway status due to accumulation of edema and swelling from sometimes injurious and traumatic airway manipulations, coupled with the ICU team’s resuscitative efforts. A brief time delay is certainly appropriate. Unfortunately, a delay of more than several hours often leads to worsening of the airway situation due to resuscitative measures and the effects of aggressive airway manipulation: edema, swelling, secretions secondary to trauma, volume administration, placement in the supine position and upper airway manipulation (e.g., nasogastric tube insertion, suctioning).
| Table 9. Complications of Delayed SAD Removal (n=63, in 61 Patients) | |
| Complication | Occurrences |
|---|---|
| Hypoxia (SpO2 <90%) | 12 |
| Severe hypoxia (SpO2 <80%) | 11 |
| Bradycardia | 4 |
| Multiple attempts (≥2 attempts) | 19 |
| Cardiac arrest | 1 |
| Rescue device needed | 10 |
| Unintended extubation of the trachea | 4 |
| Posterior pharyngeal wall erosiona | 1 |
| Urgent tracheostomyb | 1 |
|
a ILMA left in place for approximately 36 hours.
b Successful airway rescue/intubation with ILMA, but massive, expanding facial/oropharyngal edema precluded ILMA removal within the first hour after rescue. Decision made to control airway surgically.
ILMA, Intubating LMA; SAD, supraglottic airway device; SpO2, peripheral capillary oxygen saturation
|
|
Miscommunication or lack of communication was at the heart of many of these delays. It is imperative that the airway team follow up with a plan for explantation of the SAD. Of note, the airway team performing the exchange procedure rarely included the same team members who placed the SAD (n=61; Table 9). It was very typical that the airway was described as much more complicated following the delay of SAD removal. This was likely due to the responding edema and swelling from the prior complicated airway manipulation. Moreover, the ongoing resuscitation efforts of the patient for his or her primary diagnoses in the post-intubation period were aggressive. The vast majority of these cases took place during the first four years of aggressive SAD deployment as a rescue adjunct (Table 9). In each case of delayed SAD explantation, removal of the SAD was over an airway exchange catheter. Improved communication among the anesthesia airway team members and those responsible for ICU care reduced the delay in explantation.
Utilization Trends
Utilization of the SAD in its role as a rescue ventilation and intubation conduit appears to be undergoing major changes (Table 10). As presented earlier in Figure 1, the use of the SAD peaked following widespread distribution and ready access at the beside for NORA emergency intubations as well as in the OR. In our institution’s “post-ASA guideline ready” period from 1996 to 2006, the SAD was deployed, in some role, in nearly one in six NORA emergency airway interventions. VL was introduced in the mid- to late 2006 and fully deployed to NORA locations in early 2007. There was a precipitous drop in the rescue use of the SAD in 2007, to about 9% following VL deployment. In the following five-year period, its use plateaued in the 4% range.
| Table 10. SAD Deployment (N=1,461) | |||
| Time Period | Ventilation Success | Intubation Success (Blind) | Intubation Success (FFB) |
|---|---|---|---|
| 1990-1996 (pre-ASA guidelines, 1.8%) | 91.7% (n=60) | 92.6% (n=27) | 47% (n=17) |
| 1996-2006 (ASA guideline ready, 18.9%) | 96.8% (n=964) | 92.7% (n=532) | 90.9% (n=341) |
| 2006-2018 (post-VL, 4.2%) | 92.9% (n=437) | 97.2% (n=141) | 83% (n=165) |
| ASA, American Society of Anesthesiologists; FFB, flexible fiber-optic bronchoscope; VL, video laryngoscopy | |||
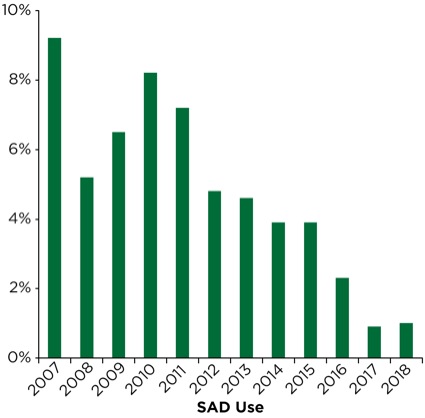
Over the past three-year period (2016-2018), our department has deployed an improved disposable DL model (BritePro, Flexicare) that may have an impact on patient care. Utilization of a video laryngoscope as a primary device or in a rescue role has changed little over three years and accounts for 50% to 60% of airway interventions. Likewise, wide distribution of disposable FFBs, in a variety of sizes, has led to a slight uptick in the role FFB plays in NORA airway management. However, the rescue role of the SAD has fallen to approximately 1% over the last two years (Figure 7).
Perhaps the SAD is being dethroned by VL and by the better-quality equipment our institution has deployed. Is VL so successful that there now is little need to deploy a SAD, store it, teach it, or even consider it? I think not. The SAD has affected the outcome of nearly 1,300 patients at our institution over many years, and has saved countless lives. I agree that there is less emphasis on the rescue role of the SAD since VL has become ubiquitous in our practice. I also have noted less emphasis of its role in the simulation teaching environment, which is now more focused on video-assisted airway management schema. Although I am aware, via our database, that patient care is much improved and hemodynamic and airway-related complications have been reduced since the advent of VL, I fear the current and next generation of airway managers may find themselves inadequately prepared to manage patients when either elective or rescue VL interventions prove unsuccessful.
VL is a wonderful addition to our armamentarium, but it also has limitations. The video laryngoscope, as an airway device, rarely fails. It is usually the operator holding it who fails through his or her limited skill set, inappropriate VL use (e.g., with trismus), and sometimes unrelenting reliance on the video laryngoscope as the one device for all cases. Further, the patient’s clinical conditions and his or her airway anatomy may affect the two steps of VL management: laryngoscopy (usually the easier step) and intubation of the trachea (usually the more difficult step).
As a backup device, the SAD has a solid record of assisting the airway team for rescue ventilation, as a conduit for intubation, and as a bridge to support the patient while preparing an alternative method or technique for securing the airway. This applies to both OR and NORA locations, the emergency department, emergency medical services and the flight crew. Of note, although the overall failure rate of VL intubation during emergency airway encounters in NORA locations is between 4% and 6% in the Hartford Hospital database, the most common rescue device, for now, remains the SAD. Thus, one should be encouraged to understand, practice and utilize the SAD for all it has to offer.
Conclusion
The SAD has a proven track record for ventilation/oxygenation and as an intubation conduit in the elective clinical setting.1,2,8,13 The roles the SAD played in the Hartford Hospital database of NORA emergency airway encounters are reflective of our society’s and others’ recommendations.1-7 The SAD afforded a consistently high success rate of effective ventilation and oxygenation under adverse conditions, given the acute and critical circumstances in the remote location. Likewise, blind and FFB-assisted SAD intubation is a highly effective method for securing the airway in a cadre of proven difficult airway patients in the NORA emergency setting. This bodes well when one compares this to Berkow’s 93% success rate of LMA Classic, AIC and FFB encounters in a mostly elective OR patient group.12 Additionally, both LMA models provided support as a provisional bridge for difficult/impossible mask ventilation. This allowed airway personnel to improve or optimize oxygenation and ventilation prior to initial or subsequent interventions, to acquire and prepare rescue adjuncts for an intervention, or to perform invasive airway access procedures with concurrent maintenance of oxygenation and ventilation.
The application of positive pressure ventilation through the SAD via a closed circuit offers several important advantages for a difficult-to-see periglottic opening that is overwhelmed and obliterated by collapsed, boggy or injured edematous mucosa. A poor view at atmospheric pressure via the open unpressurized SAD can be transformed by the application of lateralizing pressurization via a closed circuit. This salvage maneuver can be replicated by assuring ready access to a bronchoscopic adapter from the DAC.
The database highlighted an intriguing issue concerning the delayed removal of a SAD following an emergency airway rescue. It would appear that while delaying SAD removal until the patient’s clinical condition stabilizes is a reasonable step in overall patient care, the window of safety appears to be brief (one to two hours). Post-intubation changes in the airway following a traumatic intervention, coupled with use of the supine position and aggressive fluid resuscitation, probably do little to improve the status of the airway. Bronchoscopic-assisted placement of a small-caliber ETT via the LMA Classic was adequate for temporary support but clinically inappropriate for a critical ICU patient. Thus, removal of the LMA Classic, in our hands, has required the removal of the LMA Classic and ETT over an Airway Exchange Catheter (Cook Medical) followed by reintubation with a larger-caliber ETT. A distinct advantage of the LMA Classic, AIC and FFB method, although technically an exchange procedure, is that it may be accomplished more rapidly and with fewer steps than an LMA Classic–assisted intubation followed by an LMA–ETT exchange.10,12
The limitations of this review include its retrospective nature and the fact that the source is a single center with reliance on self-reporting of methods and complications taking place under sometimes extreme and adverse circumstances.
The SAD proved to be a very useful ventilation/oxygenation adjunct and intubation conduit in a variety of emergent clinical situations in NORA settings. Emergency airway management and its study remain underrepresented in the literature. Despite the extensive deployment of VL, this review supports the recommendations made by many airway management algorithms regarding and supporting the SAD as a rescue ventilation device and an intubation conduit.1-7
As always, experience and judgment using the SAD as a rescue device are paramount. Despite the impressive track record of both VL and the SAD, prudent forethought dictates that alternative rescue strategies must be immediately available in all locations where airway care is provided. Maintaining our SAD-assisted intubation skills in the simulation lab and periodically in elective surgical cases seems justified to thwart the potential extinction of its use by future generations of airway managers. For the sake of continued improvement in airway management care, we should encourage and practice SAD-assisted intubation as a trusted and reliable method of airway rescue.
References
- Cook TM, Woodall N, Frerk C, et al. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Br J Anaesth. 2011;106(5):617-631.
- Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2013;118(2):251-270.
- Berlac P, Hyldmo PK, Kongstad P, et al. Pre-hospital airway management: guidelines from a task force from the Scandinavian Society for Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2008;52(7):897-907.
- Henderson JJ, Popat MT, Latto IP, et al. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59(7):675-694.
- Baker PA, Flanagan BT, Greenland KB, et al. Equipment to manage a difficult airway during anaesthesia. Anaesth Intensive Care. 2011;39(1):16-34.
- Law JA, Broemling N, Cooper RM, et al. The difficult airway with recommendations for management. Can J Anaesth.2013;60(11):1089-1118.
- Frova G, Sorbello M. Algorithms for difficult airway management: a review. Minerva Anestesiol. 2009;75(4):201-209.
- Ferson DZ, Rosenblatt WH, Johansen MJ, et al. Use of the Intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology. 2001;95(5):1175-1181.
- Micaglio M, Ori C, Parotto M, et al. Three different approaches to fibreoptic-guided intubation via the Laryngeal Mask Airway Supreme. J Clin Anesth. 2009;21(2):153-154.
- Wong DT, Yang JJ, Mak HY, et al. Use of intubation introducers through a supraglottic airway to facilitate tracheal intubation: a brief review. Can J Anaesth. 2012;59(7):704-715.
- Cook TM, Seller C, Gupta K, et al. Non-conventional uses of the Aintree Intubation Catheter in management of the difficult airway. Anaesthesia. 2007;62(2):169-174.
- Berkow LC, Schwartz JM, Kan K, et al. Use of the Laryngeal Mask Airway-Aintree Intubating Catheter-fiberoptic bronchoscope technique for difficult intubation. J Clin Anesth. 2011;23(7):534-539.
- Baskett PJ, Parr MJ, Nolan JP. The intubating laryngeal mask. Results of a multicentre trial with experience of 500 cases. Anaesthesia. 1998;53(12):1174-1179.
- Zura A, Doyle DJ, Orlandi M. Use of the Aintree Intubation Catheter in a patient with an unexpected difficult airway. Can J Anaesth. 2005;52(6):646-649.
- Cook TM, Silsby J, Simpson TP. Airway rescue in acute upper airway obstruction using a ProSeal Laryngeal mask airway and an Aintree catheter: a review of the ProSeal Laryngeal mask airway in the management of the difficult airway. Anaesthesia. 2005;60(11):1129-1136.
- Higgs A, Clark E, Premraj K. Low-skill fibreoptic intubation: use of the Aintree Catheter with the classic LMA. Anaesthesia. 2005;60(9):915-920.
- Pothmann W, Fullekrug B, Schulte am Esch J. Fiberoptic determination of the position of the laryngeal mask [in German]. Anaesthesist. 1992;41(12):779-784.
- Atherton DP, O’Sullivan E, Lowe D, et al. A ventilation-exchange bougie for fibreoptic intubations with the laryngeal mask airway. Anaesthesia. 1996;51(12):1123-1126.
- Allison A, McCrory J. Tracheal placement of a gum elastic bougie using the laryngeal mask airway. Anaesthesia. 1990;45(5):419-420.
- Brimacombe J, Berry A. Placement of a Cook airway exchange catheter via the laryngeal mask airway. Anaesthesia. 1993;48(4):351-352.
- Chadd G, Ackers J, Bailey P. Difficult intubation aided by the laryngeal mask airway. Anaesthesia. 1989;44(12):1015.
- Murdoch JA. Emergency tracheal intubation using a gum elastic bougie through a laryngeal mask airway. Anaesthesia. 2005;60(6):626-627.
- Miller JA, Levsky ME, Givens ML, et al. Eschmann introducer through laryngeal mask airway: a cadaveric trial of an alternate means of rescue intubation. West J Emerg Med. 2010;11(1):16-19.
- Alberts ANJ. The LMA Classic™ as a conduit for tracheal intubation in adult patients: a review and practical guide. South Afr J Anaesth Analg. 2014;20(1):77-88.




Leave a Reply
You must be logged in to post a comment.