Introduction
Pectoralis major muscle (PMM) rupture is a relatively rare occurrence usually associated with strenuous physical exertion. The PMM repair has some unique anesthesia challenges associated with it. The goals for complete anesthesia during repair include both analgesia and muscle spasm control. Further, spasm control should include intraoperative muscle compliance during stretching and repairs, especially during the use of electrocauterization.
To accomplish these goals, anesthesia providers might borrow strategies more often associated with breast surgery concerning reconstruction and augmentation. Breast surgery with subpectoral implantation also involves the unique aspects of both surgical pain and muscle spasm control intraoperatively, during dissection and manipulation, and postoperatively to reduce painful involuntary contractions. Traditionally these involuntary contractions are thought to be due to the irritation and stretching of the PMM by the implant or tissue expander after surgery.
Injury to the PMM itself, outside the breast surgical realm, also appears to be on the rise. As enthusiasts of weight lifting and heavy exertion continue, and increases in construction and heavy labor are required, these unique injuries will need special attention for surgical repair, as described by Wise and colleagues.1 Thoughtful alignment of the surgical insult needs targeted anesthetic techniques that keep pain and spasm control optimized, without reliance on parenteral muscle relaxants and opioid medications.
The INTRAPEC technique has been described as an effective method of intraoperative and postoperative spasm and pain control specific to the PMM.2 We describe a case in which this novel technique, paired with an erector spinae (ESP) block, was successfully used for a pain-free, opioid-free anesthetic, which also provided appropriate spasm control for an open PMM tear repair secondary to rock climbing.
The PMM originates along the lateral sternum and inferior clavicle. The insertions of the muscle traverse the proximal humerus on the lateral region of the bicipital groove. The functions of this muscle include forward flexion, internal rotation and adduction of the shoulder. The muscle performs this action through a broad insertion originating from two distinct heads. These are the sternal and clavicular heads.3 Innervation to the PMM is by way of the median and lateral pectoral nerve. These two distinct nerves arise from the brachial plexus.
The treatment options for PMM tear include surgical repair versus nonsurgical management, primarily physical therapy. Surgery is indicated to repair a complete tear of the muscle. Nonsurgical management yields satisfactory results in patients with sedentary lifestyles because PMM weakness is generally well tolerated. The majority of PMM injuries occur secondarily to heavy lifting, and emphasis on incidence seems to be from exercise maneuvers such as bench pressing.3 Lastly, the relatively new ESP block causes analgesia to the surgical area but will not provide muscle relaxation. Other techniques would include the pectoralis plane block (PEC) 1 targeting the nerves themselves.4 This makes a single peripheral technique unlikely to be successful for both incisional pain and PMM compliance.
Literature Review
We searched for regional techniques specific to the repair and analgesia for the PMM and found a few relevant articles. The search was then expanded to include relevant anatomy and occurrence incidence in PubMed and Google Scholar. As PMM rupture is a relatively rare occurrence, relevant articles were more difficult to find, with only one meeting search parameters. The vast majority of articles were located in orthopedic journals providing background information and in case report formats. In 2000, Bak and colleagues reported that only about 150 cases could be located in the literature, supporting the notion that PMM rupture is indeed a rare occurrence.5 They suggested that surgical repair was superior in terms of functional outcome.
Kakwani and colleagues explained that in their experience of 13 incidences in their facility, 11 patients had surgical findings exactly matching the MRI reports.6 Curiously, the surgical outcomes mirrored their imaging results and surgical synergy. They reported the vast majority of causes for PMM rupture being weight lifting, specifically occurring during the bench press exercise. Of the patients who underwent surgery, 12 of the 13 had excellent/good outcomes. They reported one patient in their cohort to have had a poor outcome. This was collated following an average of two years of follow-up. The length of time required for full return of strength and activity was reported at 8.5 months, and they emphasized the importance of early activity and rehabilitation.6
Manske and Prohaska described an overview from available sources describing clinically relevant aspects of PMM rupture and repair.4 They reported that the decision to repair depended on several factors. They reported that young and active patients were likely to benefit from not only the functional return to strength and activity but also return of muscular symmetry of the chest. They reported that traditionally an anterior and lateral chest incision, needed for repair of most PMM tears, will likely blend into the delto-pectoral folds, and appear as a stretch mark. They further reported that avoidance of repair rarely led to both strength and symmetry return, but relatively sedentary individuals may not benefit from repair.7
At the time, Mooers and colleagues reported that less than 400 of these unique cases had been documented.8 They also reported that traditional operative examination revealed a tendon avulsion as the most frequent rupture type accounting for about 65% of ruptures of the PMM. Musculotendinous junction ruptures account for the majority of the ruptures outside of tendon avulsions. For those patients refusing repair or who present as unsuitable surgical candidates, the injured arm should be isolated in a sling for a period of three weeks. Following this period, physical therapy is strongly recommended.8
Merrolla and colleagues divided PMM injury into three distinct categories based on severity.9 A type 1 presentation is described as a muscle sprain. A type 2 presentation is characterized by a partial tear. A type 3 presentation is described as a complete PMM tear followed by a complete tear. Once a complete tear has been identified, it’s further subdivided according to tear location. A type 3A tear is muscular in origin. A type 3B tear is specific to the muscle belly itself. This is followed by a type 3C tear, described as a myotendinous junction injury, and a type 3D tear, described as one of tendinous origin. Lastly, a bony avulsion from the insertion has been described as a type 3E tear, and a muscle tendon substance rupture is called type 3F.9
Case Presentation
A 34-year-old man presented to the surgical area with a chief complaint of complete rupture (type 3) of the PMM occurring during rock climbing. The ASA physical status II patient weighed 99 kg, was 70 cm tall, and had a surgical history significant for tonsillectomy and wisdom tooth extraction, both performed under general anesthesia without anesthesia complications.
Based on the patient’s history and presentation and lab work, additional studies were not indicated by either the surgical team or anesthesia. After a thorough explanation regarding the risks and benefits, both the surgeon and patient were made aware that this combination of ultrasound-guided ESP and INTRAPEC had not yet been used for this purpose, but agreed to proceed.
Written consent was obtained, and the patient was placed on the OR table following moderate sedation with 2 mg of IV midazolam. Standard monitors were placed, and a uSmart 3300 (Terason) with a covered high-frequency linear probe used and image was optimized. The patient was placed in the prone position for the ESP block, and institutional timeout was performed confirming laterality. After institutional skin prep, the skin and projected needle path over the T4 transverse process were infiltrated with 5 mL of 1% lidocaine. A total of 30 mL of 0.25% ropivacaine were infiltrated, aspirating every 5 mL as originally described by Forero and colleagues.5
The patient tolerated this well and was assisted into the supine position after completion. Following full preoxygenation, general anesthesia with a laryngeal mask airway was instituted with spontaneous respiration preserved. The anesthetic state was maintained with sevoflurane, and the patient received 4 mg of ondansetron and 30 mg of ketorolac intravenously after induction of anesthesia.
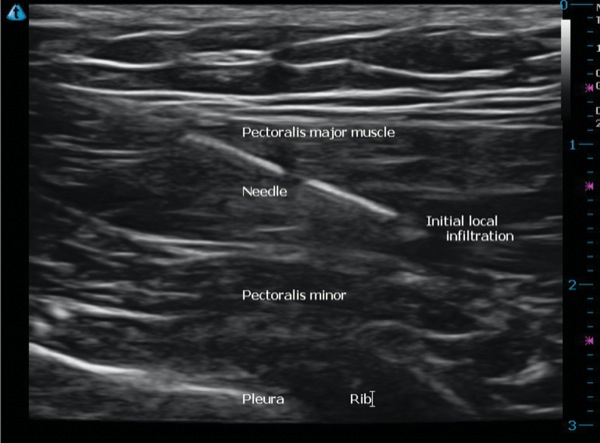
The ultrasound-guided INTRAPEC technique was then performed with the covered linear probe by first positioning it over the broadest portion of the affected PMM. This was performed in the transverse orientation. The broadest and thickest portion of the PMM was identified. This was confirmed with ultrasound visualization by positioning the probe near the nipple, and scanning along the anterior chest wall in cephalad fashion. Following institutional skin prep, the INTRAPEC injection was placed using a 4-inch block needle (B. Braun). The needle was then directed, inplane to this large portion of the PMM, completing the INTRAPEC injection as originally described by Kline.10 A total of 15 mL of 0.25% ropivacaine were then directed into the large portion of the muscle in 5-mL increments in a fanlike pattern. A flowering effect of each injection was appreciated, ensuring a saturation of the most prominent region of the muscle. See Figure 1 for graphical depiction of the ultrasound-guided INTRAPEC injection. See Figure 2 for an ultrasound example of proper needle placement for the INTRAPEC injection. The patient tolerated this procedure well, and underwent open repair of the muscle rupture without event.
On incision, no increase in heart rate, respiratory rate or blood pressure was noticed. The surgical course was uneventful and further facilitated by targeted PMM compliance, presumably caused by the INTRAPEC injection. The patient proceeded through the surgical repair and into the PACU following an uneventful emergence. It was noted that the patient was pain-free and required no parenteral muscle relaxants or opioid medications to produce intraoperative and postoperative complete analgesia and spasm control for the entire surgical course, and well into the PACU.
Conclusion
Despite PMM rupture being a relatively rare occurrence, its incidence is on the rise and has some unique anesthesia challenges associated with it. The goals of an intervention for pectoralis repair include both analgesia and spasm control. Further, spasm control should include intraoperative muscle compliance during stretching for repairs and manipulation, especially during the use of electrocauterization. Our traditional understanding of peripheral nerve blocks excludes any single injection of that would successfully meet all these goals. The combination of the ESP block and novel INTRAPEC approach was successful in this case.
The INTRAPEC injection is simple, does not require great precision (unlike that required for the PECs 1 block), and avoids the dangers of needle and local anesthetic placement within millimeters of known vasculature or known implants. However, it seems to provide appropriate intraoperative muscle compliance and analgesia. In this case presentation, we believe that this combination directly led to the omission of opioid medications traditionally needed for intraoperative and postoperative pain control for this unique surgical repair. More examples and formalized studies are required to make further conclusions about the effectiveness of this combination of ultrasound guided regional techniques for this purpose.
References
- Wise P, Gallo R. Increasing incidence of pectoralis major ruptures in NFL players. Orthop J Sports Med. 2019;7(7 suppl 5):2325967119S00396. Published online 2019 Jul 29. doi: 10.1177/2325967119S00396.
- Kline J, Lee w, Wofford K. INTRAPEC technique controls pectoralis spasm and pain for subpectoral breast implantation: a retrospective study. Plastic and Reconstructive Surgery – Global Open. 2020;8(2):e2646. doi: 10.1097/GOX.0000000000002646.
- Metzger PD, Bailey JR, Filler RD, et al. Pectoralis major muscle rupture repair: technique using unicortical buttons. Arthrosc Tech. 2012;1(1):e119-e125.
- Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621-627.
- Bak K, Ian CJ, Henderson P. Rupture of the pectoralis major: a meta-analysis of 112 cases. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):113-119.
- Kakwani RG, Matthews JJ, Kumar KM, et al. Rupture of the pectoralis major muscle: surgical treatment in athletes. Int Orthop. 2007;31(2):159-163.
- Manske RC, Prohaska D. Pectoralis major tendon repair post surgical rehabilitation. N Am J Sports Phys Ther. 2007;2(1):22-33.
- Mooers BR, Westermann RW, Wolf BR. Outcomes following suture-anchor repair of pectoralis major tears: a case series and review of the literature. Iowa Orthop J. 2015;35:8-12.
- Merolla G, Paladini P, Campi F, et al. Pectoralis major tendon rupture. Surgical procedures review. Muscles Ligaments Tendons J. 2012;2(2):96-103.
- Kline J. Ultrasound-guided INTRAPEC injection for breast surgery: a novel solution for surgical field improvement during electrocautery and implantation and for postoperative pain and muscle spasm reduction for breast surgery. Anesthesia eJournal. 2018;6:18-21. https://anesthesiaejournal.com/ index.php/ aej/ article/ view/ 87. Accessed March 2, 2020



Leave a Reply
You must be logged in to post a comment.