Anesthesiology News
Orange, California
Ehlers-Danlos syndrome is a group of inherited disorders that affect connective tissues, including blood vessel walls. Ehlers-Danlos syndrome hypermobility type III (EDS type III) can be associated with atlanto-occipital subluxation, resulting in spontaneous loss of consciousness and apnea.1 It is difficult to distinguish whether the cause is vascular insufficiency or cord compression—for example, from cervical medullary syndrome—and often requires live imaging while the coma and apnea are elicited.
After obtaining patient consent, we describe the anesthetic management of an EDS type III patient with a complex medical history who must undergo dynamic vertebral artery imaging.
Case Presentation
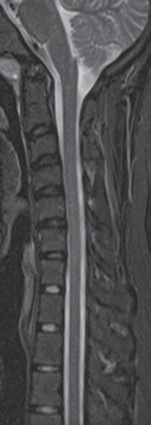
A 22-year-old man with EDS type III, status post-occipital to occipital-C2 fusion in 2015, presented for work-up of worsening simultaneous syncopal, apneic, and paralytic events, with cervical medullary syndrome and possible vertebrobasilar vascular positional insufficiency. A previous cervical spine MRI did not demonstrate any cord compression or defects (Figure 1). However, a CT scan of the neck showed cervical fusion screws abutting the vertebral arteries bilaterally, raising concern for potential vertebrobasilar insufficiency with head movement (Figure 2).
Consequently, a cerebral angiogram with dynamic vertebral artery imaging was ordered. Although the procedure has low risk, interesting anesthetic challenges were presented by the patient’s comorbid conditions: EDS type III, dystonia, hemiplegic migraine, dysautonomia, hypokalemic periodic paralysis, gene mutation for malignant hyperthermia, and recent placement of a diaphragm pacer.
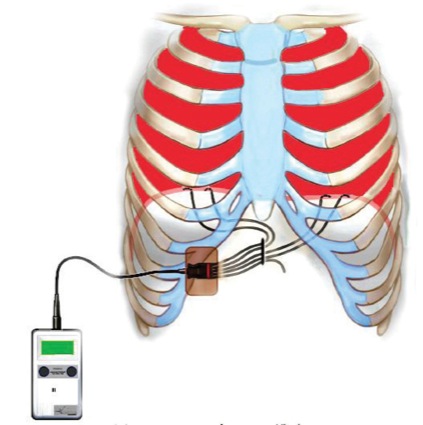
An extensive preoperative discussion took place with the patient and his family. For the past year, the patient could self-induce (and accidentally induce) loss of consciousness and apnea using the “sleeper position.” This was done by tilting his head downward followed by retropulsion. To regain consciousness and respiration, his mother would place his head in a neutral anatomic position followed by lateral rotation 30 degrees to the right or left. The patient’s mother, who used this so-called “Lazarus maneuver,” was present during the procedure. To maintain his respirations, a diaphragmatic pacer (NeuRx, Synapse Biomedical) was implanted several months before the imaging study (Figure 3). External battery packs were placed at the bedside.
In the event the diaphragmatic pacer or Lazarus maneuver failed, difficult airway equipment was readily available in the interventional radiology suite. Malignant hyperthermia precautions were taken. Two large-bore IVs and a radial arterial catheter were placed before initiation of the case. Given that the sleeper position needed to be induced during imaging, local anesthesia/monitored anesthetic care (MAC) was chosen for the procedure.
The patient underwent a cerebral angiogram with dynamic positional studies to document changes in the vertebrobasilar arterial flow with cervical movement. A face mask was placed with 100% oxygen at 6 L per minute with end-tidal carbon dioxide monitoring. After obtaining baseline right vertebral artery flow studies, the patient’s head was placed in the sleeper position. He lost consciousness and became apneic. The diaphragmatic pacer was then turned on. After a dynamic right-sided vertebral angiogram was obtained, the anesthesiology team used the Lazarus maneuver and he regained consciousness (able to blink on command) and became conversant over the next few minutes. The diaphragmatic pacer was turned off. The same process was repeated for left vertebral artery imaging.
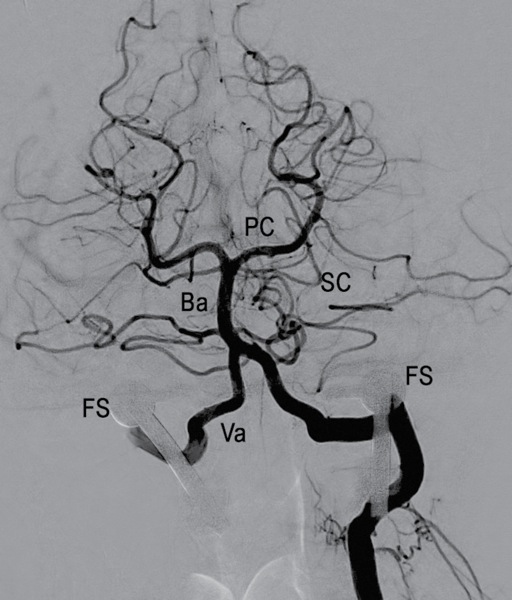
The studies demonstrated widely patent vertebral arteries with a normal codominant vertebrobasilar system at baseline and during the maneuvers (Figure 4). There was no significant occlusion caused by the cervical fusion screws adjacent to bilateral vertebral arteries (Figure 5). The patient tolerated the procedure without any sequelae. He was discharged home later that same day.
Figure 4. Selective right vertebral artery digital subtraction angiogram; lateral projection with patient in symptomatic position. Normal vertebrobasilar system without interval vessel stenosis during provocative maneuver.
Figure 5. Selective left vertebral artery digital subtraction angiogram; second order branch, transverse projection. Codominant vertebral arteries with no abnormalities noted of the vertebrobasilar system.
Discussion
EDS is an inheritable connective tissue disorder, with an incidence of 1 in 10,000 to 1 in 25,000. EDS type III is characterized by generalized joint hypermobility, recurring joint dislocations/subluxations, chronic musculoskeletal pain, and smooth/velvety skin.1-3
Preoperative workup for EDS type III should include any history of intubation difficulty; cardiac involvement (aortic root dilation, aortic or mitral valve insufficiency as determined by echocardiography); predisposition to bruising/hematoma; skeletal muscle weakness; scoliosis; autonomic dysautonomia (via tilt table or orthostatic blood pressure monitoring); and unusual responses to local anesthetic, regional nerve block, or pain medication.1-5
Patients with EDS may exhibit occipito-atlantoaxial and atlantoaxial junction instability due to increased looseness of ligaments. Atlantoaxial subluxation is the most frequent form of instability, typically from forward subluxation of C1 on C2.2 Traction and kinking of the contralateral vertebral artery may occur with lateral rotation of C1 on C2 to complete occlusion of bilateral vertebral arteries at 45 degrees from anatomic neutral position.6 Craniocervical instability can lead to basilar invagination (vertical translocation of C1 through the foramen magnum) and possible brainstem compression. Neurologic signs include headache, dysphagia, respiratory dysfunction, motor dysfunction, or even quadriparesis.2,7 Thus, in-line head stabilization in the neutral position during mask ventilation and intubation is essential.
Intraoperative airway considerations include challenging mask ventilation and difficult endotracheal tube placement caused by occipito-atlantoaxial instability, temporomandibular joint luxation, and premature spondylosis reducing mouth opening. Elective fiber-optic intubation or video laryngoscopy may be warranted. Use of a smaller-sized endotracheal tube and reduced pilot cuff pressures to avoid mucosal damage and bleeding are recommended. Peak airway pressures should be minimized to reduce the risk for pneumothorax.1-3,5
Patient positioning is of the utmost importance with EDS. Common complications include neck compression and brachial plexus stretching; joint luxation; skin injury and hematoma formation from insufficient padding or tourniquet use; and shearing forces from medical tape.1-3 Use of a noninvasive blood pressure cuff in patients who are susceptible to hematoma formation (which is still possible with repeated insufflation) and ultrasound guidance for arterial and central venous catheter placement are suggested.1-3 Although it is pathophysiologically unrelated, von Willebrand disease or thrombocytopenia may co-occur with EDS type III, thereby increasing bleeding risk.1
EDS type III patients have no special limitations regarding MAC, general, or regional anesthesia. Depolarizing and nondepolarizing neuromuscular agents can be used safely for muscle relaxation. Ultrasound guidance for regional blocks is recommended to reduce accidental vascular injuries. Topical anesthetics (lidocaine 2.5% and prilocaine 2.5% cream [EMLA]) or local infiltration may be delayed or less effective due to tissue scarring.1-3,5 Neuraxial blockade can be more challenging because of spinal anatomy changes (kyphosis, scoliosis), tissue fragility (linked to spontaneous dural rupture and post–dural puncture headache), and meningeal involvement manifested as Tarlov cysts (cerebrospinal fluid–filled perineural cysts, typically located at the sacral or thoracic regions). Preprocedural imaging can help depict possible spine pathology.1-5,8
Women with EDS who are pregnant and have known aortic root dilation should receive an echocardiogram each trimester. Primigravid patients in labor and delivery may progress rapidly (<4 hours).1,2 Cesarean delivery may be preferred to vaginally, especially in EDS type III, because the latter is associated with hip dislocation and pelvic prolapse after an episiotomy.1-5
There is an increased risk for autonomic dysfunction, particularly neurally mediated hypotension and positional orthostatic tachycardic syndrome.1-3 Early volume administration and vasopressor use with close hemodynamic monitoring are advised.1-3
Unique to this patient was use of the NeuRx diaphragmatic pacer, which is only FDA-approved for patients with amyotrophic lateral sclerosis. Nevertheless, we ensured the pacer was fully interrogated and we heeded the manufacturer’s precautions. For instance, electrocautery could potentially cause skin burns at the lead exit site and/or damage the pulse generator. Unlike cardiac pacemakers, the NeuRx pacer does not have a sensing function and so the operator must manually turn pacing on and off.
Conclusion
Anesthetic management of patients with EDS type III poses several complex issues that warrant a thorough systems-based preoperative evaluation. When evaluating for vertebrobasilar artery insufficiency or cervical medullary syndrome from cervical instability, dynamic imaging of the vertebral arteries can be performed safely under local anesthesia/MAC with careful anesthetic planning, effective communication, and vigilance.
References
- Levy HP. Hypermobile Ehlers-Danlos syndrome. Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington; 2004;1993-2018.
- Wiesmann T, Castori M, Malfait F, et al. Recommendations for anesthesia and perioperative management in patients with Ehlers-Danlos syndrome(s). Orphanet J Rare Dis. 2014;9:109.
- OrphanAnesthesia. Anesthesia recommendations for patients suffering from Ehlers-Danlos syndrome. www.orphananesthesia.eu/ en/ rare-diseases/ published-guidelines/ cat_view/ 61-rare-diseases/ 60-published-guidelines/ 89-ehlersdanlos-syndrome.html
- Arendt-Nielsen L, Kaalund S, Bjerring P, et al. Insufficient effect of local analgesics in Ehlers Danlos type III patients (connective tissue disorder). Acta Anaesthesiol Scand. 1990;34(5):358-361.
- Hakim AJ, Grahame R, Norris P, et al. Local anaesthetic failure in joint hypermobility syndrome. J Royal Soc Med. 2005;98(2):84-85.
- Menezes AH, Traynelis VC. Anatomy and biomechanics of normal craniovertebral junction and biomechanics of stabilization. Child’s Nervous System. 2008;24(10):1091-1100.
- Henderson FC, Austin C, Benzel E, et al. Neurological and spinal manifestations of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):195-211.
- Castori M, Morlino S, Celletti C, et al. Management of pain and fatigue in the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome, hypermobility type): Principles and proposal for a multidisciplinary approach. Am J Med Genet Part A. 2012;158A(8):2055-2070.




Leave a Reply
You must be logged in to post a comment.