Anesthesiology News
Departments of Anesthesiology and Critical Care & Biomedical and Health Informatics
The Children’s Hospital of Philadelphia
Philadelphia, Pennsylvania
2018 Society for Technology in Anesthesia Annual Meeting Program Co-Chair
Departments of Anesthesiology and Bioengineering
University of Utah Health
Salt Lake City, Utah
2018 Society for Technology in Anesthesia Annual Meeting Program Co-Chair
Recent advancements in tools for capturing, recording, and processing health care data have transformed health care delivery and spurred the development of new technology. Advances in computing technology, device interoperability, and electronic health records1,2 have facilitated access to increased data volume, variety, and velocity, particularly in the areas of patient monitoring, genomics, and out-of-hospital data.3
For example, progress in patient monitoring devices allows streaming of vital sign data to be collected with repeated observations. While the volume of data acquired in health care may not technically be considered “big data” and medicine is not a data science per se, there are now much more simultaneously acquired data available than ever before, which allow for a finer granularity of analysis and have the potential for uncovering more about patients, their conditions, and their care.
Clinical Data Are Central to Innovation
Whether the focus of innovation in anesthesiology and perioperative medicine is in advancing clinical research, earlier detection of disease, medical device development, real-time monitoring for patient safety, development of algorithms for predicting adverse clinical events,4-6 or improving the efficient delivery of health care,7 reliable data have always been essential. The data are needed on many levels in the innovation process, including for identifying a clinical problem, demonstrating market need, researching and developing a product, and securing regulatory approval and completing postmarket analyses.8 Furthermore, data may be crucial at all stages of the innovation process to prove the innovative endeavor is needed. In our current health care environment, defined by limited resources and a value-based approach to clinical practice, having data on hand to demonstrate a need, value, and tangible outcomes in a cost-effective manner is key for decision making.
Data as a central component of innovation was a major theme shared by several successful innovators from academic institutions at the STA meeting in a panel moderated by Clyde Matava, MBCHb, DA, MMed. One of the panelists, George Shorten, MD, PhD, from the University College Cork in Ireland, presented his group’s work at the ASSERT (Application of Science to Simulation-based Education and Research on Training) Centre. The ASSERT Centre is a unique educational and research facility that is designed to reduce medical errors and improve patient care through the deliberate training of anesthesiology and surgical skills in a high-definition simulated clinical environment. Through the use of data-driven educational metrics and goal-oriented simulation outcomes, the ASSERT Centre has demonstrated the ability to improve medical training.9-11
Recent work also was presented to improve anesthesia management by understanding patient-specific responses to propofol. Samsun Lampotang, PhD, presented his work in diving deeper into the practice of anesthesiology and bringing our field one step closer to the promises of precision medicine by showing race-specific variations in propofol-induced loss of consciousness.12 On a larger scale, research work like this has very practical applications to daily clinical practice where individual anesthesiologists can obtain more data about patient factors that drive their intraoperative management. Improved data-driven decision making can affect the quality of care delivered, reduce waste, and reduce adverse outcomes.
Of interest, innovation in anesthesiology practice is not limited to applications on Earth. Although not something often discussed in a typical conference or journal club, there is significant research being conducted on the planning and practice of anesthesia and intensive care in space. The keynote speaker at the 2018 STA meeting was Matthieu Komorowski, MD, MRes, who provided a fascinating and eye-opening presentation of the environmental, physiologic, and resource-limited challenges of providing anesthesia in space. The excitement of space travel is balanced with the harsh reality of risk. Dr. Komorowski presented the need for using mathematical modeling and simulation studies to increase data and knowledge about planning and executing anesthesia and critical care during space missions (Figure 1). It is clear that more data are needed to understand how to effectively deliver procedural care such as chest compressions and intubations, actions that may be relatively familiar and routine on Earth but not in a zero-gravity resource-constrained environment.13
Some new techniques were presented for analyzing clinical data during a session led by Patrick Tighe, MD, including a comparison between conventional machine learning and deep learning, as well as an exciting new process for medical data called reinforcement learning.14 Dr. Tighe also described the differences among data science, machine learning, and artificial intelligence: “Data science produces insights, machine learning produces predictions, and artificial intelligence produces actions.”15 Additionally, a promising new concept was described by which automatic feature extraction may be developed.16 Traditionally, feature extraction requires a person to define relevant and non-redundant values from a set of measured data in order to facilitate the learning and generalization steps in machine learning. Automation of this process may lead to more efficient dimensionality reduction for large data sets.
Data Granularity: More Data, More Insights
Increasing the volume, sophistication, and type of data collected is fueling innovation in health care delivery and our understanding of patient disease in the perioperative setting. New technologies, sensors, and tools for conducting research and patient assessment in the perioperative space can collect data with finer granularity, leading to novel insights. Catherine Price, PhD, ABPP-CN, demonstrated the value of increasing the quantity and granularity of data collected for the Clock Drawing Test.17
The Clock Drawing Test is traditionally a simple pencil and paper test that has been used for over 50 years to help assess whether a patient has cognitive impairment, such as a neurologic disorder or cognitive decline. Assessing the final image drawn by the patient and factors such as symmetry and accuracy are important in the screening test. Souillard-Mandar et al, however, are innovating the field of studying cognitive decline in perioperative patients through a modern approach using a digital pen that collects more data about the position of the pen, fine movements, pen stroke data, temporal data points, and other features that are inherent in drawing, for which a simple pencil could never provide insight.18 They then used machine learning analytical techniques to try to improve the prediction and detection of cognitive impairment. The digital pen and machine learning analytical techniques are being applied in perioperative elderly surgical patients to better understand and assess postoperative cognitive decline using higher resolution data for a test revolutionized by data and analytics.
Collaboration and Data Sharing For Innovation
The complexity of modern-day anesthesiology and perioperative medicine research; the advancement in technologies; and the specialization of almost every scientific, medical, and engineering discipline means that collaboration is essential when it comes to successful innovation. Conducting work in individual silos while not sharing data is likely to only lead to small and at best incremental innovations rather than disruptive, game-changing innovation.19,20
In fact, some suggest that disruptive innovation in a field often can come from those individuals who are actually on the outside of that field. The “Medici Effect” is a phrase that is used to describe this phenomenon in which innovation comes from the collaboration and intersection of diverse industries, cultures, and disciplines, where ideas from one field combine with those of another. This concept was named after the Medici Dynasty, a banking family in Italy in the 14th century that supported different groups of people, such as painters, poets, scientists, philosophers, architects, and others. The intersection of all the talent from different fields is considered a major contribution to innovation and the Renaissance.21 Similarly, the Medici Effect with modern-day physicians, researchers, engineers, human factors specialists, designers, and others can lead to collaboration, an intersection of ideas, and disruptive innovation in the delivery of care.
When managed prudently and transparently, a unique type of collaboration can take place between the disciplines of academia and industry. Although a lack of transparency may lead some to be concerned over conflicts of interest and bias in collaborative efforts between these 2 groups, many successful companies and advances in anesthesiology care have grown from seeds planted in collaboration between academia and industry. It is vital for industry to invest in solutions that clinicians value and support, and without a chance for dialogue with clinical thought leaders, industry leaders may easily make incorrect assumptions and take decisions that are not supported in the clinical market. The STA meeting provides a unique forum in which industry experts and academic scientists, engineers, and clinicians can gather to share their work and ideas, as well as develop pertinent industry guidelines and standards.
Interoperability and Data Security
It is the duty of health care providers and institutions to ensure the security of patient information; however, as greater volumes of data are collected and analyzed in order to improve patient care and reduce costs, the risk for significant impact from large-scale data breeches also increases.
The medical risks associated with cybersecurity include loss of a device, loss of data availability, degradation of performance, and loss of confidence in the equipment. As devices become more connected, development of standards for cybersecurity and interoperability has become increasingly important. Julian M. Goldman, MD, provided some perspective to the trade-offs of security and interoperability with a look at the work he is doing at the Medical Device Plug-and-Play Interoperability Lab at Massachusetts General Hospital and Partners HealthCare, in Boston, Massachusetts. This unique and innovative lab is at the center of testing medical device security and integration as well as clinical workflow simulations. As the internet of things and the number of devices increase in health care delivery, it will be important to ensure that devices maintain a balance between safety and accessibility. The FDA has developed a guidance document to assist medical device manufacturers to address interoperability.22
As the use of big data and device interoperability expand in the clinical domain, it will be important to draw upon relevant experiences from experts in medicine, the FDA, medical device companies, and organizations such as the Association for the Advancement of Medical Instrumentation, National Institute of Standards and Technology (NIST), CyberUL, and others to develop appropriate procedures. For example, NIST has developed a simple and powerful cybersecurity framework to identify, protect, detect, respond to, and recover from a problem.
Automated Drug Delivery
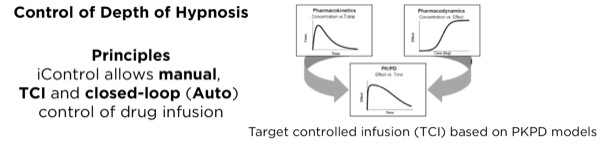
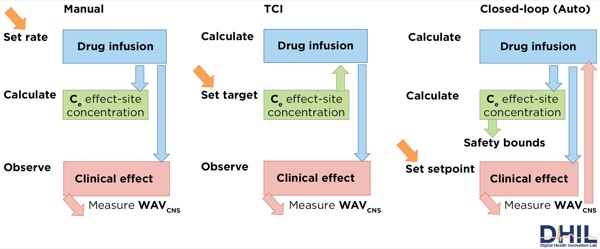
Closed-loop automation exists in many industries, including the automotive and aircraft industries. Guy Dumont, PhD, presented a survey of recent work (Figure 2) with a focus on automation in IV anesthesia.23 Dr Dumont stressed that arguably one of the most difficult issues about closed-loop control has to do with safety and preserving control. When situational awareness is maintained, a human operator can step in and resume control effectively.
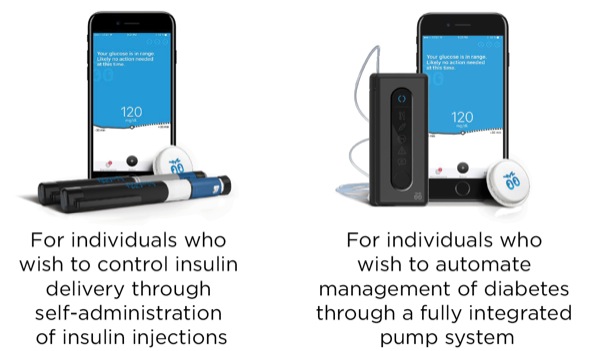
Lane Desborough, MASc, highlighted some of the burdens of living with diabetes and suggested that automation of insulin administration could relieve the burden for people with a data-driven disease in which an average person is required to self-titrate a dangerous hormone on a 24/7/365 basis. He presented current work from Bigfoot Biomedical, showing 2 insulin delivery devices (Figure 3) currently being developed. Mr. Desborough also made the case for using modeling and simulation during product development, citing advantages such as the ability to rapidly evaluate multiple algorithm candidates and parameters; the chance to simulate performance of closed-loop algorithms in a larger, more varied population; and the opportunity to predict performance over months or years of use.
Simulation testing work also allows the situational awareness and data display components of the device to be thoroughly tested. Human-centered design and interoperability will be key to the success of closed-loop delivery of anesthesia. A call was made for STA members to develop consensus statements about simulation testing requirements for closed-loop control.
Entrepreneurship, Innovation, and Translational Science
STA collaborated with the Foundation for Anesthesia Education and Research to provide a successful workshop on the last day of the meeting called “Swimming with the Sharks,” which promoted academic entrepreneurship. The workshop was moderated by Steven Shafer, MD, and dedicated to Ted Stanley, MD. The first portion of the program, “Success and Failure Travel Together,” was devoted to commercializing clinical ideas. Four panelists talked about their personal commercialization stories to challenge the common perception that a smart person with a good idea is a guaranteed success.
In the second portion of the program, several clinicians who are working to translate their ideas from bench to bedside were selected to present their 15-minute pitch to a panel of investors. The investor panel then critiqued the ideas in front of an audience to highlight the myriad of concerns an investor must consider when selecting a technology for commercialization. In addition to the common aspects of introducing the technology and discussing the clinical problem that the pitched device addresses, it was also important for the presenter to discuss the unmet clinical need, the supposed market for the device, and other factors, such as competitors and the marketing strategy.
Conclusion
Anesthesiology and perioperative medicine have improved dramatically over the past few decades and have been heralded as a medical specialty leading the way in patient safety.24 The development of safer systems, monitors, medications, and anesthetics has contributed and relied on the use of data from the beginning of development to postmarket surveillance. Data have been central to the development of innovative technologies that have made our field safer than ever before. Continued collaboration across disciplines and learning new modalities of managing big data will continue to fuel innovation and technology development in anesthesiology for years to come. As professionals who are passionate about improving the patient experience and delivery of safe anesthetic care, we need to ensure that health care policy, research, device development, and clinical practice are informed by analysis of clinical data.
The authors thank the members of the Society for Technology in Anesthesia (STA) who presented and collaborated on the work described in this article. We acknowledge and thank the previous year’s STA annual meeting program chair, Jonathan P. Wanderer, MD, MPhil; the STA immediate past president, S. Mark Poler, MD; and the STA Board for its support. We also thank Mohamed A. Rehman, MD, for his mentorship and guidance. The 2019 STA annual meeting will take place January 9-12, 2019, in Scottsdale, Arizona. See www.stahq.org for more information.
References
- Wolfe PJ. Making sense of big data. Proc Natl Acad Sci U S A. 2013;110(45):18031-18032.
- Costa FF. Big data in biomedicine. Drug Discov Today. 2014;19(4):433-440.
- Simpao AF, Ahumada LM, Rehman MA. Big data and visual analytics in anaesthesia and health care. Br J Anaesth. 2015;115(3):350-356.
- Wharam JF, Weiner JP. The promise and peril of healthcare forecasting. Am J Manag Care. 2012;18(3):e82-e85.
- Rojas CC, Patton RM, Beckerman BG. Characterizing mammography reports for health analytics. J Med Syst. 2011;35(5):1197-1210.
- Mathias JS, Agrawal A, Feinglass J, et al. Development of a 5 year life expectancy index in older adults using predictive mining of electronic health record data. J Am Med Inform Assoc. 2013;20(e1):e118-e124.
- Kheterpal S. In the land of the blind, the one-eyed man is king. Anesthesiology. 2014;120(3):523-525.
- Varkey P, Horne A, Bennet KE. Innovation in health care: a primer. Am J Med Qual. 2008;23(5):382-388.
- Shorten GD, Gallagher AG, Satava RM. The medical procedure pathway: creating a global standard methodology to benefit patients. Eur J Anaesthesiol. 2015;32(2):79-82.
- Ahmed OM, O’Donnell BD, Gallagher AG, et al. Construct validity of a novel assessment tool for ultrasound-guided axillary brachial plexus block. Anaesthesia. 2016;71(11):1324-1331.
- Ahmed OMA, Azher I, Gallagher AG, et al. Deliberate practice using validated metrics improves skill acquisition in performance of ultrasound-guided peripheral nerve block in a simulated setting. J Clin Anesth. 2018;48:22-27.
- Lampotang S, Lizdas DE, Derendorf H, et al. Race-specific pharmacodynamic model of propofol-induced loss of consciousness. J Clin Pharmacol. 2016;56(9):1141-1150.
- Komorowski M, Fleming S. Intubation after rapid sequence induction performed by non-medical personnel during space exploration missions: a simulation pilot study in a Mars analogue environment. Extrem Physiol Med. 2015;4:19.
- Williams RJ. Simple statistical gradient-following algorithms for connectionist reinforcement learning. In: Reinforcement Learning. Boston, MA: Springer; 1992:5-32.
- Robinson D. Variance explained. http://varianceexplained.org/?r/?ds-ml-ai/?. Accessed August 6, 2018.
- Saeys Y, Inza I, Larrañaga P. A review of feature selection techniques in bioinformatics. Bioinformatics. 2007;23(19):2507-2517.
- Shulman KI, Shedletsky R, Silver IL. The challenge of time: clock-drawing and cognitive function in the elderly. Int J Geriatr Psychiatry. 1986;1(2):135-140.
- Souillard-Mandar W, Davis R, Rudin C, et al. Learning classification models of cognitive conditions from subtle behaviors in the digital Clock Drawing Test. Mach Learn. 2016;102(3):393-441.
- Bhatti Y, del Castillo J, Olson K, et al. Putting humans at the center of health care innovation. Harvard Business Review. https://hbr.org/?2018/?03/?putting-humans-at-the-center-of-health-care-innovation. March 2, 2018. Accessed June 10, 2018.
- Chiasson M, Reddy M, Kaplan B, et al. Expanding multi-disciplinary approaches to healthcare information technologies: what does information systems offer medical informatics? Int J Med Inform. 2007;76(suppl 1):S89-S97.
- Johansson F. The Medici Effect: What Elephants and Epidemics Can Teach Us About Innovation. Boston, MA: Harvard Business School Press; 2004.
- https://www.fda.gov/?medicaldevices/?digitalhealth/?ucm512245.htm. Accessed August 6, 2018.
- Dumont GA, Ansermino JM. Closed-loop control of anesthesia: a primer for anesthesiologists. Anesth Analg. 2013;117(5):1130-1138.
- Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507.



Leave a Reply
You must be logged in to post a comment.